Ku-0063794
mTOR pathway inhibitor; Inhibits mTORC1 and mTORC2 complexes
概要
Ku-0063794 is a cell-permeable, selective inhibitor of the serine-threonine kinase mammalian target of rapamycin (mTOR), inhibiting both the mTORC1 and mTORC2 complexes (IC₅₀ = 10 nM). It shows good specificity (> 1000-fold) against 76 other protein kinases or 7 lipid kinases, including PI3 kinases (García-Martínez et al.).
MAINTENANCE
· Extends the lifespan of Toll-like receptor (TLR)-activated dendritic cells by preserving mitochondrial oxidative phosphorylation (Amiel et al.).
CANCER RESEARCH
· Inhibits cell growth by inducing G1-cell cycle arrest in mouse embryonic fibroblasts and human non-small cell lung carcinoma cell lines (García-Martínez et al.; Fei et al.).
· Inhibits tumor growth in a xenograft model of renal cell carcinoma (Zhang et al.).
· Reduces keloid (fibroproliferative dermal lesion) volume in an ex vivo keloid organ culture model, and inhibits keloid cell spreading, proliferation, migration, and invasive properties in vitro (Syed et al.).
MAINTENANCE
· Extends the lifespan of Toll-like receptor (TLR)-activated dendritic cells by preserving mitochondrial oxidative phosphorylation (Amiel et al.).
CANCER RESEARCH
· Inhibits cell growth by inducing G1-cell cycle arrest in mouse embryonic fibroblasts and human non-small cell lung carcinoma cell lines (García-Martínez et al.; Fei et al.).
· Inhibits tumor growth in a xenograft model of renal cell carcinoma (Zhang et al.).
· Reduces keloid (fibroproliferative dermal lesion) volume in an ex vivo keloid organ culture model, and inhibits keloid cell spreading, proliferation, migration, and invasive properties in vitro (Syed et al.).
Alternative Names
Not applicable
Cell Type
Cancer Cells and Cell Lines, Dendritic Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Maintenance
Area of Interest
Cancer Research
CAS Number
938440-64-3
Chemical Formula
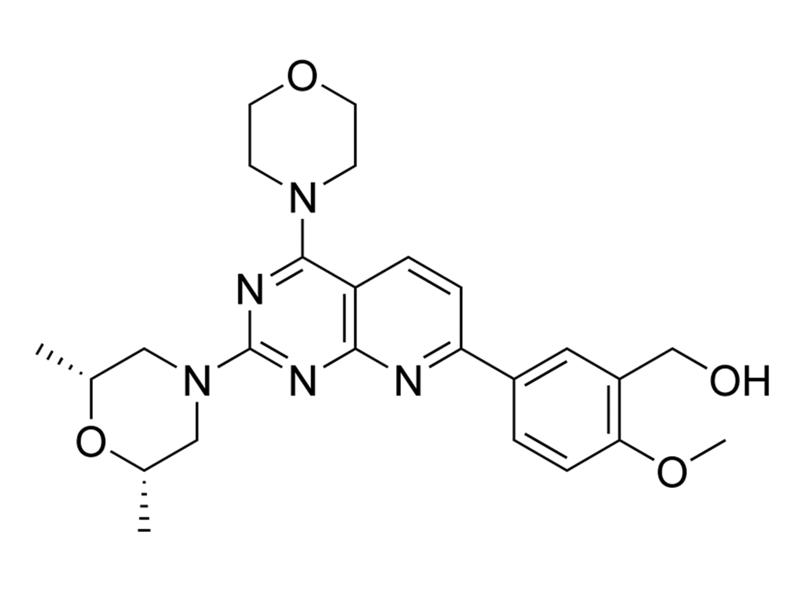
C₂₅H₃₁N₅O₄
Molecular Weight
465.5 g/mol
Purity
≥ 98%
Pathway
mTOR
Target
mTOR
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Ku-0063794 | 73232, 73234 | All | English |
| Safety Data Sheet | Ku-0063794 | 73232, 73234 | All | English |
数据及文献
Publications (5)
Journal of immunology (Baltimore, Md. : 1950) 2014
Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function.
Abstract
Abstract
TLR-mediated activation of dendritic cells (DCs) is associated with a metabolic transition in which mitochondrial oxidative phosphorylation is inhibited by endogenously synthesized NO and the cells become committed to glucose and aerobic glycolysis for survival. We show that inhibition of mechanistic target of rapamycin (mTOR) extends the lifespan of TLR-activated DCs by inhibiting the induction of NO production, thereby allowing the cells to continue to use their mitochondria to generate ATP, and allowing them the flexibility to use fatty acids or glucose as nutrients to fuel core metabolism. These data provide novel mechanistic insights into how mTOR modulates DC metabolism and cellular longevity following TLR activation and provide an explanation for previous findings that mTOR inhibition enhances the efficacy of DCs in autologous vaccination.
The Journal of investigative dermatology 2013
Potent dual inhibitors of TORC1 and TORC2 complexes (KU-0063794 and KU-0068650) demonstrate in vitro and ex vivo anti-keloid scar activity.
Abstract
Abstract
Mammalian target of rapamycin (mTOR) is essential in controlling several cellular functions. This pathway is dysregulated in keloid disease (KD). KD is a common fibroproliferative dermal lesion with an ill-defined treatment strategy. KD demonstrates excessive matrix deposition, angiogenesis, and inflammatory cell infiltration. In KD, both total and phosphorylated forms of mTOR and p70(S6K)(Thr421/Ser424) are upregulated. Therefore, the aim of this study was to investigate adenosine triphosphate-competitive inhibitors of mTOR kinase previously unreported in keloid and their comparative efficacy with Rapamycin. Here, we present two mTOR kinase inhibitors, KU-0063794 and KU-0068650, that target both mTORC1 and mTORC2 signaling. Treatment with either KU-0063794 or KU-0068650 resulted in complete suppression of Akt, mTORC1, and mTORC2, and inhibition of keloid cell spreading, proliferation, migration, and invasive properties at a very low concentration (2.5 μmol l(-1)). Both KU-0063794 and KU-0068650 significantly (Ptextless0.05) inhibited cell cycle regulation and HIF1-α expression compared with that achieved with Rapamycin alone. In addition, both compounds induced shrinkage and growth arrest in KD, associated with the inhibition of angiogenesis, induction of apoptosis, and reduction in keloid phenotype-associated markers. In contrast, Rapamycin induced minimal antitumor activity. In conclusion, potent dual mTORC1 and mTORC2 inhibitors display therapeutic potential for the treatment of KD.
PloS one 2013
A comparison of Ku0063794, a dual mTORC1 and mTORC2 inhibitor, and temsirolimus in preclinical renal cell carcinoma models.
Abstract
Abstract
Rapamycin analogs, temsirolimus and everolimus, are approved for the treatment of advance renal cell carcinoma (RCC). Currently approved agents inhibit mechanistic target of rapamycin (mTOR) complex 1 (mTORC1). However, the mTOR kinase exists in two distinct multiprotein complexes, mTORC1 and mTORC2, and both complexes may be critical regulators of cell metabolism, growth and proliferation. Furthermore, it has been proposed that drug resistance develops due to compensatory activation of mTORC2 signaling during treatment with temsirolimus or everolimus. We evaluated Ku0063794, which is a small molecule that inhibits both mTOR complexes. Ku0063794 was compared to temsirolimus in preclinical models for renal cell carcinoma. Ku0063794 was effective in inhibiting the phosphorylation of signaling proteins downstream of both mTORC1 and mTORC2, including p70 S6K, 4E-BP1 and Akt. Ku0063794 was more effective than temsirolimus in decreasing the viability and growth of RCC cell lines, Caki-1 and 786-O, in vitro by inducing cell cycle arrest and autophagy, but not apoptosis. However, in a xenograft model there was no difference in the inhibition of tumor growth by Ku0063794 or temsirolimus. A potential explanation is that temsirolimus has additional effects on the tumor microenvironment. Consistent with this possibility, temsirolimus, but not Ku0063794, decreased tumor angiogenesis in vivo, and decreased the viability of HUVEC (Human Umbilical Vein Endothelial Cells) cells in vitro at pharmacologically relevant concentrations. Furthermore, expression levels of VEGF and PDGF were lower in Caki-1 and 786-O cells treated with temsirolimus than cells treated with Ku0063794.
PloS one 2013
Targeting mTOR to overcome epidermal growth factor receptor tyrosine kinase inhibitor resistance in non-small cell lung cancer cells.
Abstract
Abstract
AIMS: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have shown dramatic clinical benefits in advanced non-small cell lung cancer (NSCLC); however, resistance remains a serious problem in clinical practice. The present study analyzed mTOR-associated signaling-pathway differences between the EGFR TKI-sensitive and -resistant NSCLC cell lines and investigated the feasibility of targeting mTOR with specific mTOR inhibitor in EGFR TKI resistant NSCLC cells. METHODS: We selected four different types of EGFR TKI-sensitive and -resistant NSCLC cells: PC9, PC9GR, H1650 and H1975 cells as models to detect mTOR-associated signaling-pathway differences by western blot and Immunoprecipitation and evaluated the antiproliferative effect and cell cycle arrest of ku-0063794 by MTT method and flow cytometry. RESULTS: In the present study, we observed that mTORC2-associated Akt ser473-FOXO1 signaling pathway in a basal state was highly activated in resistant cells. In vitro mTORC1 and mTORC2 kinase activities assays showed that EGFR TKI-resistant NSCLC cell lines had higher mTORC2 kinase activity, whereas sensitive cells had higher mTORC1 kinase activity in the basal state. The ATP-competitive mTOR inhibitor ku-0063794 showed dramatic antiproliferative effects and G1-cell cycle arrest in both sensitive and resistant cells. Ku-0063794 at the IC50 concentration effectively inhibited both mTOR and p70S6K phosphorylation levels; the latter is an mTORC1 substrate and did not upregulate Akt ser473 phosphorylation which would be induced by rapamycin and resulted in partial inhibition of FOXO1 phosphorylation. We also observed that EGFR TKI-sensitive and -resistant clinical NSCLC tumor specimens had higher total and phosphorylated p70S6K expression levels. CONCLUSION: Our results indicate mTORC2-associated signaling-pathway was hyperactivated in EGFR TKI-resistant cells and targeting mTOR with specific mTOR inhibitors is likely a good strategy for patients with EGFR mutant NSCLC who develop EGFR TKI resistance; the potential specific roles of mTORC2 in EGFR TKI-resistant NSCLC cells were still unknown and should be further investigated.
The Biochemical journal 2009
Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR).
Abstract
Abstract
mTOR (mammalian target of rapamycin) stimulates cell growth by phosphorylating and promoting activation of AGC (protein kinase A/protein kinase G/protein kinase C) family kinases such as Akt (protein kinase B), S6K (p70 ribosomal S6 kinase) and SGK (serum and glucocorticoid protein kinase). mTORC1 (mTOR complex-1) phosphorylates the hydrophobic motif of S6K, whereas mTORC2 phosphorylates the hydrophobic motif of Akt and SGK. In the present paper we describe the small molecule Ku-0063794, which inhibits both mTORC1 and mTORC2 with an IC50 of approximately 10 nM, but does not suppress the activity of 76 other protein kinases or seven lipid kinases, including Class 1 PI3Ks (phosphoinositide 3-kinases) at 1000-fold higher concentrations. Ku-0063794 is cell permeant, suppresses activation and hydrophobic motif phosphorylation of Akt, S6K and SGK, but not RSK (ribosomal S6 kinase), an AGC kinase not regulated by mTOR. Ku-0063794 also inhibited phosphorylation of the T-loop Thr308 residue of Akt phosphorylated by PDK1 (3-phosphoinositide-dependent protein kinase-1). We interpret this as implying phosphorylation of Ser473 promotes phosphorylation of Thr308 and/or induces a conformational change that protects Thr308 from dephosphorylation. In contrast, Ku-0063794 does not affect Thr308 phosphorylation in fibroblasts lacking essential mTORC2 subunits, suggesting that signalling processes have adapted to enable Thr308 phosphorylation to occur in the absence of Ser473 phosphorylation. We found that Ku-0063794 induced a much greater dephosphorylation of the mTORC1 substrate 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) than rapamycin, even in mTORC2-deficient cells, suggesting a form of mTOR distinct from mTORC1, or mTORC2 phosphorylates 4E-BP1. Ku-0063794 also suppressed cell growth and induced a G1-cell-cycle arrest. Our results indicate that Ku-0063794 will be useful in delineating the physiological roles of mTOR and may have utility in treatment of cancers in which this pathway is inappropriately activated.