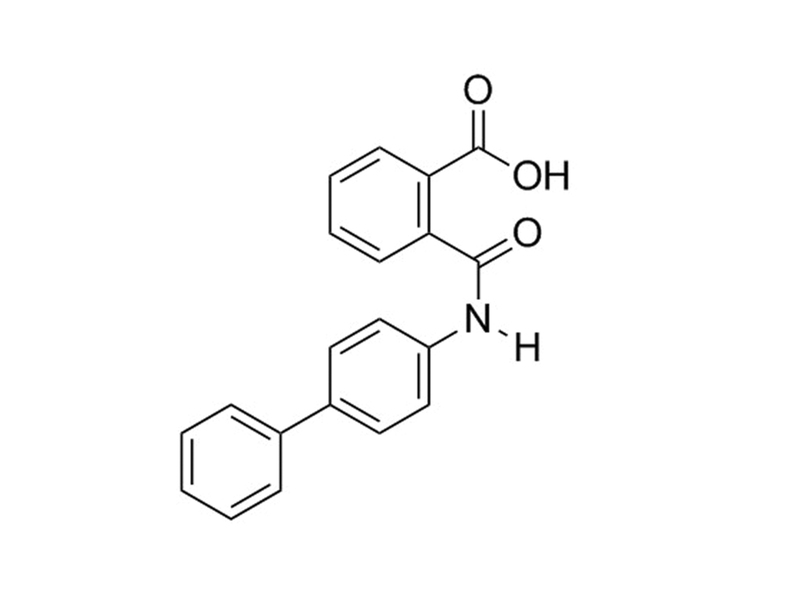
Kartogenin
RUNX1 transcriptional activator; Binds filamin A
概要
Kartogenin induces chondrogenesis by binding the actin-binding protein, filamin A, which disrupts its interaction with the transcription factor core-binding factor β subunit (CBFβ). When dissociated from filamin A, CBFβ translocates to the nucleus and forms a transcriptional complex with the runt-related transcription factor RUNX1, which enables chondrocyte differentiation (Johnson et al.).
DIFFERENTIATION
· Promotes differentiation of human bone marrow mesenchymal stem cells into chondrocytes (Johnson et al.; Zhang et al.)
· Promotes cartilage formation/repair in mouse models of osteoarthritis or when injected into mouse tendon-bone junctions (Johnson et al.; Zhang et al.)
· Promotes type-I collagen synthesis in human dermal fibroblasts in vitro and in the dermis of mice through activation of the SMAD4/SMAD5 pathway (Wang et al.).
DIFFERENTIATION
· Promotes differentiation of human bone marrow mesenchymal stem cells into chondrocytes (Johnson et al.; Zhang et al.)
· Promotes cartilage formation/repair in mouse models of osteoarthritis or when injected into mouse tendon-bone junctions (Johnson et al.; Zhang et al.)
· Promotes type-I collagen synthesis in human dermal fibroblasts in vitro and in the dermis of mice through activation of the SMAD4/SMAD5 pathway (Wang et al.).
Alternative Names
Not applicable
Cell Type
Chondrocytes, Mesenchymal Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Disease Modeling, Stem Cell Biology
CAS Number
4727-31-5
Chemical Formula
C₂₀H₁₅NO₃
Molecular Weight
317.3 g/mol
Purity
≥ 98%
Target
Filamin A
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Kartogenin | 72572 | All | English |
| Safety Data Sheet | Kartogenin | 72572 | All | English |
数据及文献
Publications (3)
Bone research 2014 MAY
Kartogenin induces cartilage-like tissue formation in tendon-bone junction.
Abstract
Abstract
Tendon-bone junctions (TBJs) are frequently injured, especially in athletic settings. Healing of TBJ injuries is slow and is often repaired with scar tissue formation that compromises normal function. This study explored the feasibility of using kartogenin (KGN), a biocompound, to enhance the healing of injured TBJs. We first determined the effects of KGN on the proliferation and chondrogenic differentiation of rabbit bone marrow stromal cells (BMSCs) and patellar tendon stem/progenitor cells (PTSCs) in vitro. KGN enhanced cell proliferation in both cell types in a concentration-dependent manner and induced chondrogenic differentiation of stem cells, as demonstrated by high expression levels of chondrogenic markers aggrecan, collagen II and Sox-9. Besides, KGN induced the formation of cartilage-like tissues in cell cultures, as observed through the staining of abundant proteoglycans, collagen II and osteocalcin. When injected into intact rat patellar tendons in vivo, KGN induced cartilage-like tissue formation in the injected area. Similarly, when KGN was injected into experimentally injured rat Achilles TBJs, wound healing in the TBJs was enhanced, as evidenced by the formation of extensive cartilage-like tissues. These results suggest that KGN may be used as an effective cell-free clinical therapy to enhance the healing of injured TBJs.
Biochemical and biophysical research communications 2014 JUL
A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway.
Abstract
Abstract
Declined production of collagen by fibroblasts is one of the major causes of aging appearance. However, only few of compounds found in cosmetic products are able to directly increase collagen synthesis. A novel small heterocyclic compound called kartogenin (KGN) was found to stimulate collagen synthesis of mesenchymal stem cells (MSCs). So, we hypothesized and tested that if KGN could be applied to stimulate the collagen synthesis of fibroblasts. Human dermal fibroblasts in vitro were treated with various concentrations of KGN, with dimethyl sulfoxide (DMSO) serving as the negative control. Real-time reverse-transcription polymerase chain reaction, Western blot, and immunofluorescence analyses were performed to examine the expression of collagen and transforming growth factor beta (TGF-β) signaling pathway. The production of collagen was also tested in vivo by Masson's trichrome stain and immunohistochemistry in the dermis of mice administrated with KGN. Results showed that without obvious influence on fibroblasts' apoptosis and viability, KGN stimulated type-I collagen synthesis of fibroblasts at the mRNA and protein levels in a time-dependent manner, but KGN did not induce expression of α-skeletal muscle actin (α-sma) or matrix metallopeptidase1 (MMP1), MMP9 in vitro. Smad4/smad5 of the TGF-β signaling pathway was activated by KGN while MAPK signaling pathway remained unchanged. KGN also increased type-I collagen synthesis in the dermis of BALB/C mice. Our results indicated that KGN promoted the type-I collagen synthesis of dermal fibroblasts in vitro and in the dermis of mice through activation of the smad4/smad5 pathway. This molecule could be used in wound healing, tissue engineering of fibroblasts, or aesthetic and reconstructive procedures.
Science 2012 MAY
A stem cell-based approach to cartilage repair.
Abstract
Abstract
Osteoarthritis (OA) is a degenerative joint disease that involves the destruction of articular cartilage and eventually leads to disability. Molecules that promote the selective differentiation of multipotent mesenchymal stem cells (MSCs) into chondrocytes may stimulate the repair of damaged cartilage. Using an image-based high-throughput screen, we identified the small molecule kartogenin, which promotes chondrocyte differentiation (median effective concentration = 100 nM), shows chondroprotective effects in vitro, and is efficacious in two OA animal models. Kartogenin binds filamin A, disrupts its interaction with the transcription factor core-binding factor β subunit (CBFβ), and induces chondrogenesis by regulating the CBFβ-RUNX1 transcriptional program. This work provides new insights into the control of chondrogenesis that may ultimately lead to a stem cell-based therapy for osteoarthritis.