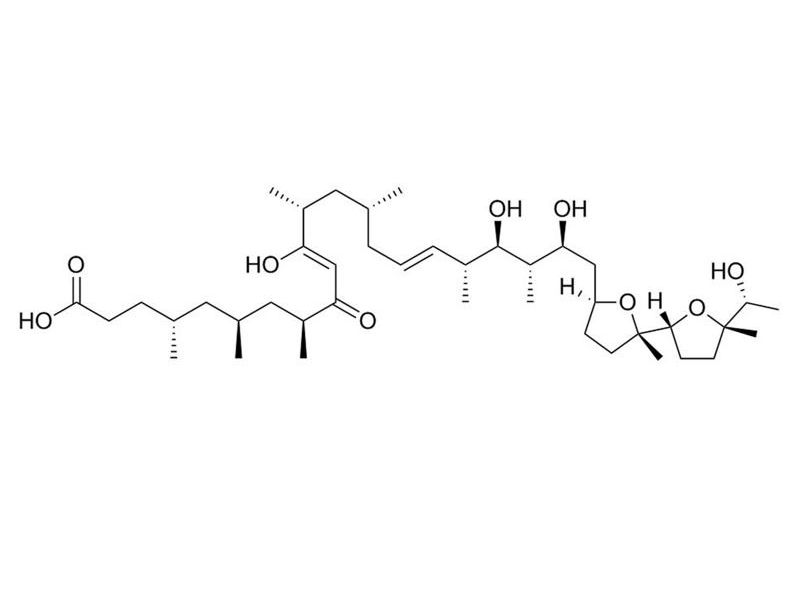
Ionomycin
Calcium ionophore
概要
Ionomycin is a potent and selective calcium ionophore derived from Streptomyces conglobatus (Liu et al.). It is used as a research tool to rapidly raise the intracellular level of calcium, and to study calcium transport across biological membranes by inducing the release of cytosolic calcium stores (Morgan & Jacob; Yoshida & Plant). Ionomycin is a more effective Ca++ ionophore than A23187, but less effective at binding and carrying Mg++ (Liu & Hermann). Ionomycin is able to activate and prime the polymorphonuclear neutrophil (PMN) oxidase (Elzi et al.), and is used in conjunction with Phorbol 12-myristate 13-acetate (PMA; Catalog #74042) for the activation of T cells (IC₅₀ = 5.8 nM; Caraher et al.; Zhang et al.). This product is supplied as a 10 mg/mL solution in ethanol.
IMMUNOLOGY
· Activates T cells from human, mouse, or rat sources, in combination with PMA, to express cytokines including IL-17, IL-4, IL-10, and IL-2 (Caraher et al.; Harrington et al.; Parrish-Novak et al.).
IMMUNOLOGY
· Activates T cells from human, mouse, or rat sources, in combination with PMA, to express cytokines including IL-17, IL-4, IL-10, and IL-2 (Caraher et al.; Harrington et al.; Parrish-Novak et al.).
Alternative Names
SQ 23377
Cell Type
T Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Activation
Area of Interest
Immunology
CAS Number
56092-81-0; 64-17-5
Chemical Formula
C₄₁H₇₂O₉
Molecular Weight
709 g/mol
Purity
≥ 98%
Pathway
Calcium Signaling
Target
Calcium
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | Ionomycin | 73722, 73724 | Lot BX29879 or higher | English |
| Product Information Sheet 2 | Ionomycin | 73722, 73724 | Lot BX29711 or lower | English |
| Safety Data Sheet | Ionomycin | 73722, 73724 | All | English |
数据及文献
Publications (9)
Nature immunology 2005 NOV
Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.
Abstract
Abstract
CD4(+) T cells producing interleukin 17 (IL-17) are associated with autoimmunity, although the precise mechanisms that control their development are undefined. Here we present data that challenge the idea of a shared developmental pathway with T helper type 1 (T(H)1) or T(H)2 lineages and instead favor the idea of a distinct effector lineage we call 'T(H)-17'. The development of T(H)-17 cells from naive precursor cells was potently inhibited by interferon-gamma (IFN-gamma) and IL-4, whereas committed T(H)-17 cells were resistant to suppression by T(H)1 or T(H)2 cytokines. In the absence of IFN-gamma and IL-4, IL-23 induced naive precursor cells to differentiate into T(H)-17 cells independently of the transcription factors STAT1, T-bet, STAT4 and STAT6. These findings provide a basis for understanding how inhibition of IFN-gamma signaling enhances development of pathogenic T(H)-17 effector cells that can exacerbate autoimmunity.
American journal of physiology. Cell physiology 2001 JUL
Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils.
Abstract
Abstract
Many receptor-linked agents that prime or activate the NADPH oxidase in polymorphonuclear neutrophils (PMNs) elicit changes in cytosolic Ca2+ concentration and activate mitogen-activated protein (MAP) kinases. To investigate the role of Ca2+ in the activation of p38 and p42/44 MAP kinases, we examined the effects of the Ca2+-selective ionophore ionomycin on priming and activation of the PMN oxidase. Ionomycin caused a rapid rise in cytosolic Ca2+ that was due to both a release of cytosolic Ca2+ stores and Ca2+ influx. Ionomycin also activated (2 microM) and primed (20-200 nM) the PMN oxidase. Dual phosphorylation of p38 MAP kinase and phosphorylation of its substrate activating transcription factor-2 were detected at ionomycin concentrations that prime or activate the PMN oxidase, while dual phosphorylation of p42/44 MAP kinase and phosphorylation of its substrate Elk-1 were elicited at 0.2-2 microM. SB-203580, a p38 MAP kinase antagonist, inhibited ionomycin-induced activation of the oxidase (68 +/- 8%, P textless 0.05) and tyrosine phosphorylation of 105- and 72-kDa proteins; conversely, PD-98059, an inhibitor of MAP/extracellular signal-related kinase 1, had no effect. Treatment of PMNs with thapsigargin resulted in priming of the oxidase and activation of p38 MAP kinase. Chelation of cytosolic but not extracellular Ca2+ completely inhibited ionomycin activation of p38 MAP kinase, whereas chelation of extracellular Ca2+ abrogated activation of p42/44 MAP kinase. These results demonstrate the importance of changes in cytosolic Ca2+ for MAP kinase activation in PMNs.
Journal of immunological methods 2000 OCT
Flow cytometric analysis of intracellular IFN-gamma, IL-4 and IL-10 in CD3(+)4(+) T-cells from rat spleen.
Abstract
Abstract
The application of multi-parameter flow cytometry for the assessment of T-cell and cytokine functioning has been used by several groups for studying human and mouse samples, although little has been reported for the rat. Here we report the optimisation of immunofluorescent staining for cell surface and intracellular antigens using three-colour flow cytometric analysis to measure the frequency of rat CD3(+)4(+) T-cells that produce IFN-gamma, IL-4 and IL-10. In vitro stimulation of IFN-gamma production required incubation of splenocytes with PMA and ionomycin in the presence of the protein transport inhibitor brefeldin A for 6 h. Three stimulation protocols for IL-4 and IL-10 production were evaluated. In vitro priming of splenic T-cells with antibodies against CD3 and CD28 and recombinant cytokines (IL-2 and IL-4) for 5 days followed by restimulation with PMA and ionomycin was required to stimulate cells to produce either IL-4 or IL-10. Brefeldin A was found to be a more suitable protein transport inhibitor than monensin. This method will be useful for analysing the nature of individual rat cytokine-producing cells in a variety of experimental model systems.
Nature 2000 NOV
Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function.
Abstract
Abstract
Cytokines are important in the regulation of haematopoiesis and immune responses, and can influence lymphocyte development. Here we have identified a class I cytokine receptor that is selectively expressed in lymphoid tissues and is capable of signal transduction. The full-length receptor was expressed in BaF3 cells, which created a functional assay for ligand detection and cloning. Conditioned media from activated human CD3+ T cells supported proliferation of the assay cell line. We constructed a complementary DNA expression library from activated human CD3+ T cells, and identified a cytokine with a four-helix-bundle structure using functional cloning. This cytokine is most closely related to IL2 and IL15, and has been designated IL21 with the receptor designated IL21 R. In vitro assays suggest that IL21 has a role in the proliferation and maturation of natural killer (NK) cell populations from bone marrow, in the proliferation of mature B-cell populations co-stimulated with anti-CD40, and in the proliferation of T cells co-stimulated with anti-CD3.
Life sciences 1997 JAN
Antiproliferative and immunosuppressive properties of microcolin A, a marine-derived lipopeptide.
Abstract
Abstract
The immunosuppressive effects of microcolin A, a lipopeptide extracted from the marine blue green alga Lyngbya majuscula were investigated. Microcolin A suppressed concanavalin A (IC50 = 5.8 nM), phytohemagglutinin (IC50 = 12.5 nM) and lipopolysaccharide (IC50 = 8.0 nM) induced proliferation of murine splenocytes. Mixed lymphocyte reaction (IC50 = 5.0 nM), anti-IgM (mu-chain specific) (IC50 = 10.0 nM), and phorbol 12-myristate 13-acetate plus ionomycin (IC50 = 5.8 nM) stimulation of murine splenocytes were all similarly suppressed by microcolin A. The inhibitory activity of microcolin A was time-dependent and reversible and was not associated with a reduction in cell viability. Moreover, microcolin A not only inhibited IL-2 production and IL-2 receptor expression by concanavalin A activated splenocytes, but also suppressed in vitro antibody responsiveness to keyhole limpet hemocyanin. These results indicate that microcolin A is a potent immunosuppressive and antiproliferative agent.
The Biochemical journal 1994 JUN
Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane.
Abstract
Abstract
In fura-2-loaded ECV304 cells ionomycin elicited a saturable biphasic change in intracellular Ca2+ concentration ([Ca2+]i), where the initial phase represented mobilization of intracellular stores and the sustained component represented Ca2+ influx. To examine whether ionomycin could stimulate influx via a store-dependent mechanism. Mn2+ entry was monitored by the quenching of fura-2 fluorescence: influx was enhanced even after ionomycin wash-out, provided that internal stores were not refilled with Ca2+. Moreover, the maximal rate of histamine-stimulated Mn2+ entry was unaffected by ionomycin, suggesting a common route of entry. The Ca(2+)-entry blocker SK&F 96365 inhibited both the ionomycin-induced Mn2+ entry and the sustained [Ca2+]i response to the ionophore (leaving the initial peak [Ca2+]i response unaffected). In other experiments, although addition of ionomycin further increased the plateau phase induced by 100 microM histamine, the increase was completely abolished by pretreatment with the store Ca(2+)-ATPase inhibitor cyclopiazonic acid (CPA). Furthermore, in store-depleted cells, re-addition of 1 mM extracellular Ca2+ (in the presence of CPA plus histamine) led to a rapid rise in [Ca2+]i, dependent on Ca2+ influx, with kinetics that were not enhanced by ionomycin. These data suggest that ionomycin acts primarily at the level of the internal Ca2+ stores, so that, at the concentrations used here (textless or = 1 microM), it increases Ca2+ (and Mn2+) influx via activation of endogenous entry pathways and not by plasmalemmal translocation.