IWR-1-endo
WNT pathway inhibitor; AXIN2 stabilizer
概要
IWR-1-endo potently inhibits WNT signaling by blocking a cell-based WNT/β-catenin pathway reporter response with an IC₅₀ value of 180 nM (Chen et al.). It inhibits WNT-induced accumulation of β-catenin, through stabilization of the destruction complex member AXIN2 (Chen et al.).
MAINTENANCE AND SELF-RENEWAL
· Promotes self-renewal and maintains pluripotency of human embryonic stem cells and mouse Epi-stem cells when used in combination with CHIR99021 (Kim et al.).
DIFFERENTIATION
· Promotes differentiation of cardiomyocytes from human pluripotent stem cells (PSCs) that have been induced to mesoderm by addition of Activin A and/or BMP4 (Ren et al.; Willems et al.)
· Induces the differentiation of human PSC-derived alveolar epithelial type II (AETII) to AETI cells (Ghaedi et al.).
MAINTENANCE AND SELF-RENEWAL
· Promotes self-renewal and maintains pluripotency of human embryonic stem cells and mouse Epi-stem cells when used in combination with CHIR99021 (Kim et al.).
DIFFERENTIATION
· Promotes differentiation of cardiomyocytes from human pluripotent stem cells (PSCs) that have been induced to mesoderm by addition of Activin A and/or BMP4 (Ren et al.; Willems et al.)
· Induces the differentiation of human PSC-derived alveolar epithelial type II (AETII) to AETI cells (Ghaedi et al.).
Alternative Names
Not applicable
Cell Type
Airway Cells, Cardiomyocytes, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Expansion, Maintenance
Area of Interest
Epithelial Cell Biology, Stem Cell Biology
CAS Number
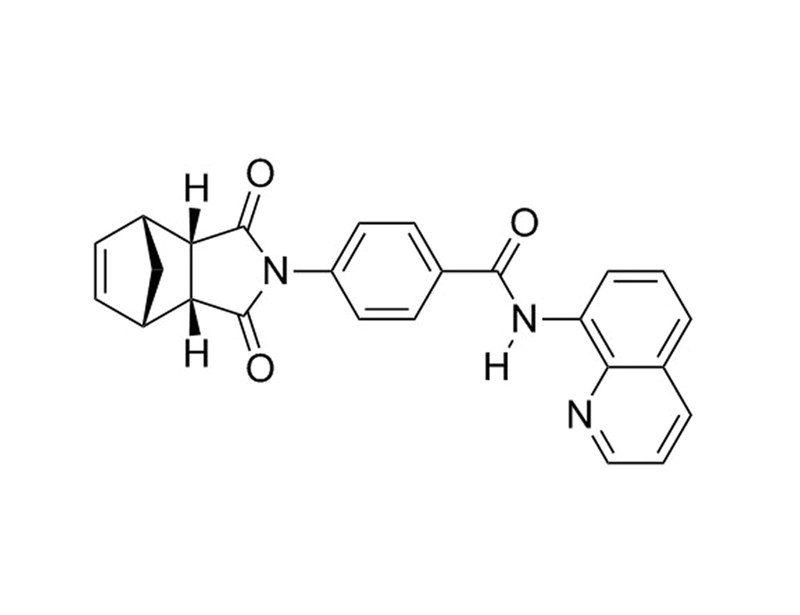
1127442-82-3
Chemical Formula
C₂₅H₁₉N₃O₃
Molecular Weight
409.4 g/mol
Purity
≥ 98%
Pathway
WNT
Target
Axin
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | IWR-1-endo | 72562, 72564 | All | English |
| Safety Data Sheet | IWR-1-endo | 72562, 72564 | All | English |
数据及文献
Publications (8)
The Journal of clinical investigation 2013 NOV
Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix.
Abstract
Abstract
The use of induced pluripotent stem cells (iPSCs) has been postulated to be the most effective strategy for developing patient-specific respiratory epithelial cells, which may be valuable for lung-related cell therapy and lung tissue engineering. We generated a relatively homogeneous population of alveolar epithelial type II (AETII) and type I (AETI) cells from human iPSCs that had phenotypic properties similar to those of mature human AETII and AETI cells. We used these cells to explore whether lung tissue can be regenerated in vitro. Consistent with an AETII phenotype, we found that up to 97% of cells were positive for surfactant protein C, 95% for mucin-1, 93% for surfactant protein B, and 89% for the epithelial marker CD54. Additionally, exposing induced AETII to a Wnt/β-catenin inhibitor (IWR-1) changed the iPSC-AETII-like phenotype to a predominantly AETI-like phenotype. We found that of induced AET1 cells, more than 90% were positive for type I markers, T1α, and caveolin-1. Acellular lung matrices were prepared from whole rat or human adult lungs treated with decellularization reagents, followed by seeding these matrices with alveolar cells derived from human iPSCs. Under appropriate culture conditions, these progenitor cells adhered to and proliferated within the 3D lung tissue scaffold and displayed markers of differentiated pulmonary epithelium.
Nature communications 2013 JAN
Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal.
Abstract
Abstract
Wnt/β-catenin signalling has a variety of roles in regulating stem cell fates. Its specific role in mouse epiblast stem cell self-renewal, however, remains poorly understood. Here we show that Wnt/β-catenin functions in both self-renewal and differentiation in mouse epiblast stem cells. Stabilization and nuclear translocation of β-catenin and its subsequent binding to T-cell factors induces differentiation. Conversely, retention of stabilized β-catenin in the cytoplasm maintains self-renewal. Cytoplasmic retention of β-catenin is effected by stabilization of Axin2, a downstream target of β-catenin, or by genetic modifications to β-catenin that prevent its nuclear translocation. We also find that human embryonic stem cell and mouse epiblast stem cell fates are regulated by β-catenin through similar mechanisms. Our results elucidate a new role for β-catenin in stem cell self-renewal that is independent of its transcriptional activity and will have broad implications in understanding the molecular regulation of stem cell fate.
Journal of molecular and cellular cardiology 2011 SEP
Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells.
Abstract
Abstract
Human induced pluripotent stem (iPS) cells potentially provide a unique resource for generating patient-specific cardiomyocytes to study cardiac disease mechanisms and treatments. However, existing approaches to cardiomyocyte production from human iPS cells are inefficient, limiting the application of iPS cells in basic and translational cardiac research. Furthermore, strategies to accurately record changes in iPS cell-derived cardiomyocyte action potential duration (APD) are needed to monitor APD-related cardiac disease and for rapid drug screening. We examined whether modulation of the bone morphogenetic protein 4 (BMP-4) and Wnt/β-catenin signaling pathways could induce efficient cardiac differentiation of human iPS cells. We found that early treatment of human iPS cells with BMP-4 followed by late treatment with small molecule Wnt inhibitors led to a marked increase in production of cardiomyocytes compared to existing differentiation strategies. Using immunocytochemical staining and real-time intracellular calcium imaging, we showed that these induced cardiomyocytes expressed typical sarcomeric markers, exhibited normal rhythmic Ca(2+) transients, and responded to both β-adrenergic and electric stimulation. Furthermore, human iPS cell-derived cardiomyocytes demonstrated characteristic changes in action potential duration in response to cardioactive drugs procainamide and verapamil using voltage-sensitive dye-based optical recording. Thus, modulation of the BMP-4 and Wnt signaling pathways in human iPS cells leads to highly efficient production of cardiomyocytes with typical electrophysiological function and pharmacologic responsiveness. The use of human iPS cell-derived cardiomyocytes and the application of calcium- and voltage-sensitive dyes for the direct, rapid measurement of iPS cell-derived cardiomyocyte activity promise to offer attractive platforms for studying cardiac disease mechanisms and therapeutics.
Circulation research 2011 AUG
Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm.
Abstract
Abstract
RATIONALE: Human embryonic stem cells can form cardiomyocytes when cultured under differentiation conditions. Although the initiating step of mesoderm formation is well characterized, the subsequent steps that promote for cardiac lineages are poorly understood and limit the yield of cardiomyocytes. OBJECTIVE: Our aim was to develop a human embryonic stem cell-based high-content screening assay to discover small molecules that drive cardiogenic differentiation after mesoderm is established to improve our understanding of the biology involved. Screening of libraries of small-molecule pathway modulators was predicted to provide insight into the cellular proteins and signaling pathways that control stem cell cardiogenesis. METHODS AND RESULTS: Approximately 550 known pathway modulators were screened in a high-content screening assay, with hits being called out by the appearance of a red fluorescent protein driven by the promoter of the cardiac-specific MYH6 gene. One potent small molecule was identified that inhibits transduction of the canonical Wnt response within the cell, which demonstrated that Wnt inhibition alone was sufficient to generate cardiomyocytes from human embryonic stem cell-derived mesoderm cells. Transcriptional profiling of inhibitor-treated compared with vehicle-treated samples further indicated that inhibition of Wnt does not induce other mesoderm lineages. Notably, several other Wnt inhibitors were very efficient in inducing cardiogenesis, including a molecule that prevents Wnts from being secreted by the cell, which confirmed that Wnt inhibition was the relevant biological activity. CONCLUSIONS: Pharmacological inhibition of Wnt signaling is sufficient to drive human mesoderm cells to form cardiomyocytes; this could yield novel tools for the benefit of pharmaceutical and clinical applications.
Nature chemical biology 2009 FEB
Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer.
Abstract
Abstract
The pervasive influence of secreted Wnt signaling proteins in tissue homeostasis and tumorigenesis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. By screening a diverse synthetic chemical library, we have discovered two new classes of small molecules that disrupt Wnt pathway responses; whereas one class inhibits the activity of Porcupine, a membrane-bound acyltransferase that is essential to the production of Wnt proteins, the other abrogates destruction of Axin proteins, which are suppressors of Wnt/beta-catenin pathway activity. With these small molecules, we establish a chemical genetic approach for studying Wnt pathway responses and stem cell function in adult tissue. We achieve transient, reversible suppression of Wnt/beta-catenin pathway response in vivo, and we establish a mechanism-based approach to target cancerous cell growth. The signal transduction mechanisms shown here to be chemically tractable additionally contribute to Wnt-independent signal transduction pathways and thus could be broadly exploited for chemical genetics and therapeutic goals.
Cell 2006 NOV
Wnt/beta-catenin signaling in development and disease.
Abstract
Abstract
A remarkable interdisciplinary effort has unraveled the WNT (Wingless and INT-1) signal transduction cascade over the last two decades. Wnt genes encode small secreted proteins that are found in all animal genomes. Wnt signaling is involved in virtually every aspect of embryonic development and also controls homeostatic self-renewal in a number of adult tissues. Germline mutations in the Wnt pathway cause several hereditary diseases, and somatic mutations are associated with cancer of the intestine and a variety of other tissues.