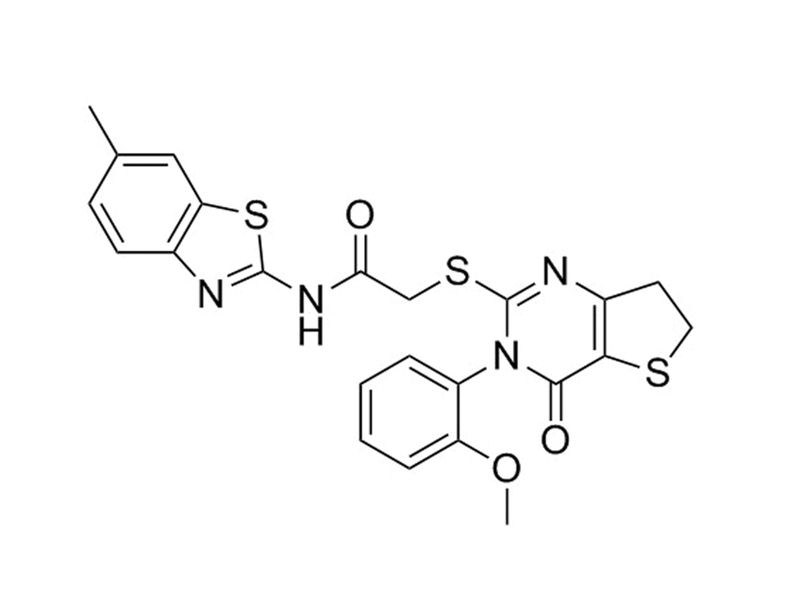
IWP-4
WNT pathway inhibitor; Inhibits Porcupine
概要
Inhibitor of WNT Production-4 (IWP-4) inhibits WNT signaling by inactivating Porcupine, a membrane-bound O-acyltransferase responsible for palmitoylating WNT proteins, which is essential for WNT signaling ability and secretion (Chen et al.). IWP-4 impairs WNT pathway activity in vitro with an IC₅₀ value of 25 nM (Chen et al.).
DIFFERENTIATION
· Promotes cardiomyocyte differentiation in human pluripotent stem cells after treatment with CHIR99021 (Lian et al., 2012, 2013; Sequiera et al.).
· Promotes cardiomyocyte differentiation in human embryonic stem cells following primitive streak induction with BMP4 and Activin A (Hudson et al.).
DIFFERENTIATION
· Promotes cardiomyocyte differentiation in human pluripotent stem cells after treatment with CHIR99021 (Lian et al., 2012, 2013; Sequiera et al.).
· Promotes cardiomyocyte differentiation in human embryonic stem cells following primitive streak induction with BMP4 and Activin A (Hudson et al.).
Alternative Names
Inhibitor of Wnt Production-4
Cell Type
Cardiomyocytes, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
686772-17-8
Chemical Formula
C₂₃H₂₀N₄O₃S₃
Molecular Weight
496.6 g/mol
Purity
≥ 95%
Pathway
WNT
Target
Porcupine
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | IWP-4 | 72552, 72554 | All | English |
| Product Information Sheet 2 | IWP-4 | 72552, 72554 | All | English |
| Safety Data Sheet | IWP-4 | 72552, 72554 | All | English |
数据及文献
Publications (9)
Methods in molecular biology (Clifton, N.J.) 2016 JAN
A Simple Protocol for the Generation of Cardiomyocytes from Human Pluripotent Stem Cells.
Abstract
Abstract
Efficient generation of cardiomyocytes from pluripotent stem cells (PSCs) for multiple downstream applications such as regenerative medicine, disease modeling, and drug screening remains a challenge. Cardiomyogenesis may be regulated in vitro by a controlled differentiation process, which involves various signaling molecules and extracellular environment. Here, we describe a simple method to efficiently generate cardiomyocytes from human embryonic stem cells and human induced pluripotent stem cells.
Stem cell reviews 2014 APR
Differentiation of human epidermal neural crest stem cells (hEPI-NCSC) into virtually homogenous populations of dopaminergic neurons.
Abstract
Abstract
Here we provide a protocol for the directed differentiation of hEPI-NCSC into midbrain dopaminergic neurons, which degenerate in Parkinson's disease. hEPI-NCSC are neural crest-derived multipotent stem cells that persist into adulthood in the bulge of hair follicles. The experimental design is distinctly different from conventional protocols for embryonic stem cells and induced pluripotent stem (iPS) cells. It includes pre-differentiation of the multipotent hEPI-NCSC into neural stem cell-like cells, followed by ventralizing, patterning, continued exposure to the TGFβ receptor inhibitor, SB431542, and at later stages of differentiation the presence of the WNT inhibitor, IWP-4. All cells expressed A9 midbrain dopaminergic neuron progenitor markers with gene expression levels comparable to those in normal human substantia nigra. The current study shows for the first time that virtually homogeneous populations of dopaminergic neurons can be derived ex vivo from somatic stem cells without the need for purification, with useful timeliness and high efficacy. This novel development is an important first step towards the establishment of fully functional dopaminergic neurons from an ontologically relevant stem cell type, hEPI-NCSC.
Nature protocols 2013 JAN
Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions.
Abstract
Abstract
The protocol described here efficiently directs human pluripotent stem cells (hPSCs) to functional cardiomyocytes in a completely defined, growth factor- and serum-free system by temporal modulation of regulators of canonical Wnt signaling. Appropriate temporal application of a glycogen synthase kinase 3 (GSK3) inhibitor combined with the expression of β-catenin shRNA or a chemical Wnt inhibitor is sufficient to produce a high yield (0.8-1.3 million cardiomyocytes per cm(2)) of virtually pure (80-98%) functional cardiomyocytes in 14 d from multiple hPSC lines without cell sorting or selection. Qualitative (immunostaining) and quantitative (flow cytometry) characterization of differentiated cells is described to assess the expression of cardiac transcription factors and myofilament proteins. Flow cytometry of BrdU incorporation or Ki67 expression in conjunction with cardiac sarcomere myosin protein expression can be used to determine the proliferative capacity of hPSC-derived cardiomyocytes. Functional human cardiomyocytes differentiated via these protocols may constitute a potential cell source for heart disease modeling, drug screening and cell-based therapeutic applications.
Stem cells and development 2012 JUN
Primitive cardiac cells from human embryonic stem cells.
Abstract
Abstract
Pluripotent stem cell-derived cardiomyocytes are currently being investigated for in vitro human heart models and as potential therapeutics for heart failure. In this study, we have developed a differentiation protocol that minimizes the need for specific human embryonic stem cell (hESC) line optimization. We first reduced the heterogeneity that exists within the starting population of bulk cultured hESCs by using cells adapted to single-cell passaging in a 2-dimensional (2D) culture format. Compared with bulk cultures, single-cell cultures comprised larger fractions of TG30(hi)/OCT4(hi) cells, corresponding to an increased expression of pluripotency markers OCT4 and NANOG, and reduced expression of early lineage-specific markers. A 2D temporal differentiation protocol was then developed, aimed at reducing the inherent heterogeneity and variability of embryoid body-based protocols, with induction of primitive streak cells using bone morphogenetic protein 4 and activin A, followed by cardiogenesis via inhibition of Wnt signaling using the small molecules IWP-4 or IWR-1. IWP-4 treatment resulted in a large percentage of cells expressing low amounts of cardiac myosin heavy chain and expression of early cardiac progenitor markers ISL1 and NKX2-5, thus indicating the production of large numbers of immature cardiomyocytes (˜65,000/cm(2) or ˜1.5 per input hESC). This protocol was shown to be effective in HES3, H9, and, to a lesser, extent, MEL1 hESC lines. In addition, we observed that IWR-1 induced predominantly atrial myosin light chain (MLC2a) expression, whereas IWP-4 induced expression of both atrial (MLC2a) and ventricular (MLC2v) forms. The intrinsic flexibility and scalability of this 2D protocol mean that the output population of primitive cardiomyocytes will be particularly accessible and useful for the investigation of molecular mechanisms driving terminal cardiomyocyte differentiation, and potentially for the future treatment of heart failure.
Proceedings of the National Academy of Sciences of the United States of America 2012 JUL
Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling.
Abstract
Abstract
Human pluripotent stem cells (hPSCs) offer the potential to generate large numbers of functional cardiomyocytes from clonal and patient-specific cell sources. Here we show that temporal modulation of Wnt signaling is both essential and sufficient for efficient cardiac induction in hPSCs under defined, growth factor-free conditions. shRNA knockdown of β-catenin during the initial stage of hPSC differentiation fully blocked cardiomyocyte specification, whereas glycogen synthase kinase 3 inhibition at this point enhanced cardiomyocyte generation. Furthermore, sequential treatment of hPSCs with glycogen synthase kinase 3 inhibitors followed by inducible expression of β-catenin shRNA or chemical inhibitors of Wnt signaling produced a high yield of virtually (up to 98%) pure functional human cardiomyocytes from multiple hPSC lines. The robust ability to generate functional cardiomyocytes under defined, growth factor-free conditions solely by genetic or chemically mediated manipulation of a single developmental pathway should facilitate scalable production of cardiac cells suitable for research and regenerative applications.
Nature chemical biology 2009 FEB
Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer.
Abstract
Abstract
The pervasive influence of secreted Wnt signaling proteins in tissue homeostasis and tumorigenesis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. By screening a diverse synthetic chemical library, we have discovered two new classes of small molecules that disrupt Wnt pathway responses; whereas one class inhibits the activity of Porcupine, a membrane-bound acyltransferase that is essential to the production of Wnt proteins, the other abrogates destruction of Axin proteins, which are suppressors of Wnt/beta-catenin pathway activity. With these small molecules, we establish a chemical genetic approach for studying Wnt pathway responses and stem cell function in adult tissue. We achieve transient, reversible suppression of Wnt/beta-catenin pathway response in vivo, and we establish a mechanism-based approach to target cancerous cell growth. The signal transduction mechanisms shown here to be chemically tractable additionally contribute to Wnt-independent signal transduction pathways and thus could be broadly exploited for chemical genetics and therapeutic goals.