IWP-3
WNT pathway inhibitor; Inhibits Porcupine
概要
Inhibitor of WNT Production-3 (IWP-3) inhibits WNT signaling by inactivating Porcupine, a membrane-bound O-acyltransferase responsible for palmitoylating WNT proteins, which is essential for WNT signaling ability and secretion (Chen et al.). IWP-3 impairs WNT pathway activity in vitro with an IC₅₀ value of 40 nM (Chen et al.).
DIFFERENTIATION
· Promotes cardiomyocyte differentiation from human embryonic stem cells that have been induced to mesoderm by addition of BMP4 and Activin A (Willems et al.).
DIFFERENTIATION
· Promotes cardiomyocyte differentiation from human embryonic stem cells that have been induced to mesoderm by addition of BMP4 and Activin A (Willems et al.).
Alternative Names
Inhibitor of Wnt Production-3
Cell Type
Cardiomyocytes, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
687561-60-0
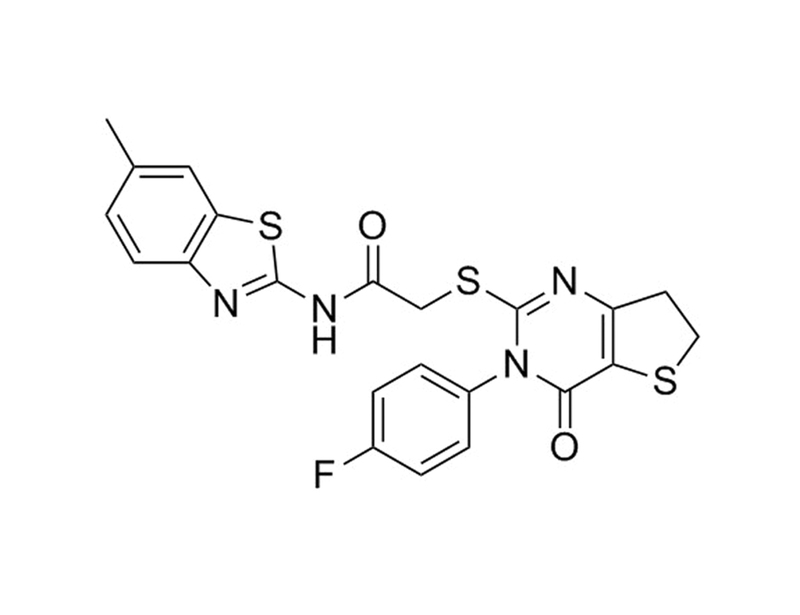
Chemical Formula
C₂₂H₁₇FN₄O₂S₃
Molecular Weight
484.6 g/mol
Purity
≥ 98%
Pathway
WNT
Target
Porcupine
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | IWP-3 | 72542, 72544 | All | English |
| Safety Data Sheet | IWP-3 | 72542, 72544 | All | English |
数据及文献
Publications (5)
Circulation research 2011 AUG
Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm.
Abstract
Abstract
RATIONALE: Human embryonic stem cells can form cardiomyocytes when cultured under differentiation conditions. Although the initiating step of mesoderm formation is well characterized, the subsequent steps that promote for cardiac lineages are poorly understood and limit the yield of cardiomyocytes. OBJECTIVE: Our aim was to develop a human embryonic stem cell-based high-content screening assay to discover small molecules that drive cardiogenic differentiation after mesoderm is established to improve our understanding of the biology involved. Screening of libraries of small-molecule pathway modulators was predicted to provide insight into the cellular proteins and signaling pathways that control stem cell cardiogenesis. METHODS AND RESULTS: Approximately 550 known pathway modulators were screened in a high-content screening assay, with hits being called out by the appearance of a red fluorescent protein driven by the promoter of the cardiac-specific MYH6 gene. One potent small molecule was identified that inhibits transduction of the canonical Wnt response within the cell, which demonstrated that Wnt inhibition alone was sufficient to generate cardiomyocytes from human embryonic stem cell-derived mesoderm cells. Transcriptional profiling of inhibitor-treated compared with vehicle-treated samples further indicated that inhibition of Wnt does not induce other mesoderm lineages. Notably, several other Wnt inhibitors were very efficient in inducing cardiogenesis, including a molecule that prevents Wnts from being secreted by the cell, which confirmed that Wnt inhibition was the relevant biological activity. CONCLUSIONS: Pharmacological inhibition of Wnt signaling is sufficient to drive human mesoderm cells to form cardiomyocytes; this could yield novel tools for the benefit of pharmaceutical and clinical applications.
Nature chemical biology 2009 FEB
Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer.
Abstract
Abstract
The pervasive influence of secreted Wnt signaling proteins in tissue homeostasis and tumorigenesis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. By screening a diverse synthetic chemical library, we have discovered two new classes of small molecules that disrupt Wnt pathway responses; whereas one class inhibits the activity of Porcupine, a membrane-bound acyltransferase that is essential to the production of Wnt proteins, the other abrogates destruction of Axin proteins, which are suppressors of Wnt/beta-catenin pathway activity. With these small molecules, we establish a chemical genetic approach for studying Wnt pathway responses and stem cell function in adult tissue. We achieve transient, reversible suppression of Wnt/beta-catenin pathway response in vivo, and we establish a mechanism-based approach to target cancerous cell growth. The signal transduction mechanisms shown here to be chemically tractable additionally contribute to Wnt-independent signal transduction pathways and thus could be broadly exploited for chemical genetics and therapeutic goals.
Cell 2006 NOV
Wnt/beta-catenin signaling in development and disease.
Abstract
Abstract
A remarkable interdisciplinary effort has unraveled the WNT (Wingless and INT-1) signal transduction cascade over the last two decades. Wnt genes encode small secreted proteins that are found in all animal genomes. Wnt signaling is involved in virtually every aspect of embryonic development and also controls homeostatic self-renewal in a number of adult tissues. Germline mutations in the Wnt pathway cause several hereditary diseases, and somatic mutations are associated with cancer of the intestine and a variety of other tissues.
Nature 2005 APR
Wnt signalling in stem cells and cancer.
Abstract
Abstract
The canonical Wnt cascade has emerged as a critical regulator of stem cells. In many tissues, activation of Wnt signalling has also been associated with cancer. This has raised the possibility that the tightly regulated self-renewal mediated by Wnt signalling in stem and progenitor cells is subverted in cancer cells to allow malignant proliferation. Insights gained from understanding how the Wnt pathway is integrally involved in both stem cell and cancer cell maintenance and growth in the intestinal, epidermal and haematopoietic systems may serve as a paradigm for understanding the dual nature of self-renewal signals.
Genes & development 2000 AUG
Wnt signaling and cancer.
Abstract