ISX-9
Inducer of neural differentiation
概要
ISX-9 is a small molecule inducer of adult neural stem cell differentiation both in vitro and in vivo (Schneider et al.). It has been shown to act through a calcium-activated signaling pathway dependent on myocyte-enhancer factor 2 (MEF2)-dependent gene expression (Schneider et al.; Petrik et al.).
REPROGRAMMING
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, Forskolin , SB431542, and I-BET151 (Li et al.).
DIFFERENTIATION
· Induces neuronal differentiation in the HCN hippocampal neural stem/progenitor cell line from adult rat, in whole brain or subventricular zone neural progenitor cells from adult mice, and in P19 embryonic carcinoma cells (Schneider et al.).
· Improves hippocampal neurogenesis and function in mice (Petrik et al.).
· Stimulates cardiac muscle gene expression and cell cycle activity in adult mouse myocardium (Russell et al.).
· Blocks tumor cell proliferation and induces neuronal gene expression in malignant astrocytes (Zhang et al.).
· Improves β-cell function, increases expression of transcription factors that enhance β-cell differentiation and increases intracellular insulin content in primary human islet cultures (Dioum et al.).
REPROGRAMMING
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, Forskolin , SB431542, and I-BET151 (Li et al.).
DIFFERENTIATION
· Induces neuronal differentiation in the HCN hippocampal neural stem/progenitor cell line from adult rat, in whole brain or subventricular zone neural progenitor cells from adult mice, and in P19 embryonic carcinoma cells (Schneider et al.).
· Improves hippocampal neurogenesis and function in mice (Petrik et al.).
· Stimulates cardiac muscle gene expression and cell cycle activity in adult mouse myocardium (Russell et al.).
· Blocks tumor cell proliferation and induces neuronal gene expression in malignant astrocytes (Zhang et al.).
· Improves β-cell function, increases expression of transcription factors that enhance β-cell differentiation and increases intracellular insulin content in primary human islet cultures (Dioum et al.).
Alternative Names
Isoxazole 9; Neuronal Differentiation Inducer III; ISX-1
Cell Type
Cancer Cells and Cell Lines, Cardiomyocytes, PSC-Derived, Neural Stem and Progenitor Cells, Neurons, Pancreatic Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Reprogramming
Area of Interest
Cancer Research, Epithelial Cell Biology, Neuroscience, Stem Cell Biology
CAS Number
832115-62-5
Chemical Formula
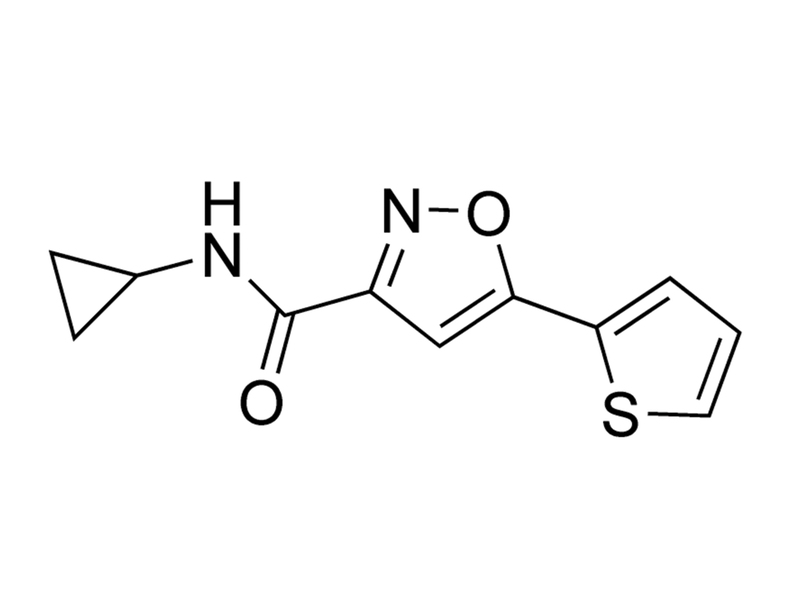
C₁₁H₁₀N₂O₂S
Molecular Weight
234.3 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | ISX-9 | 73202 | All | English |
| Safety Data Sheet | ISX-9 | 73202 | All | English |
数据及文献
Publications (6)
Cell stem cell 2015 AUG
Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons.
Abstract
Abstract
Recently, direct reprogramming between divergent lineages has been achieved by the introduction of regulatory transcription factors. This approach may provide alternative cell resources for drug discovery and regenerative medicine, but applications could be limited by the genetic manipulation involved. Here, we show that mouse fibroblasts can be directly converted into neuronal cells using only a cocktail of small molecules, with a yield of up to textgreater90% being TUJ1-positive after 16 days of induction. After a further maturation stage, these chemically induced neurons (CiNs) possessed neuron-specific expression patterns, generated action potentials, and formed functional synapses. Mechanistically, we found that a BET family bromodomain inhibitor, I-BET151, disrupted the fibroblast-specific program, while the neurogenesis inducer ISX9 was necessary to activate neuron-specific genes. Overall, our findings provide a proof of principle" for chemically induced direct reprogramming of somatic cell fates across germ layers without genetic manipulation�
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2012
Functional and mechanistic exploration of an adult neurogenesis-promoting small molecule.
Abstract
Abstract
Adult neurogenesis occurs throughout life in the mammalian hippocampus and is essential for memory and mood control. There is significant interest in identifying ways to promote neurogenesis and ensure maintenance of these hippocampal functions. Previous work with a synthetic small molecule, isoxazole 9 (Isx-9), highlighted its neuronal-differentiating properties in vitro. However, the ability of Isx-9 to drive neurogenesis in vivo or improve hippocampal function was unknown. Here we show that Isx-9 promotes neurogenesis in vivo, enhancing the proliferation and differentiation of hippocampal subgranular zone (SGZ) neuroblasts, and the dendritic arborization of adult-generated dentate gyrus neurons. Isx-9 also improves hippocampal function, enhancing memory in the Morris water maze. Notably, Isx-9 enhances neurogenesis and memory without detectable increases in cellular or animal activity or vascularization. Molecular exploration of Isx-9-induced regulation of neurogenesis (via FACS and microarray of SGZ stem and progenitor cells) suggested the involvement of the myocyte-enhancer family of proteins (Mef2). Indeed, transgenic-mediated inducible knockout of all brain-enriched Mef2 isoforms (Mef2a/c/d) specifically from neural stem cells and their progeny confirmed Mef2's requirement for Isx-9-induced increase in hippocampal neurogenesis. Thus, Isx-9 enhances hippocampal neurogenesis and memory in vivo, and its effects are reliant on Mef2, revealing a novel cell-intrinsic molecular pathway regulating adult neurogenesis.
ACS chemical biology 2012
Targeting native adult heart progenitors with cardiogenic small molecules.
Abstract
Abstract
Targeting native progenitors with small molecule pharmaceuticals that direct cell fate decisions is an attractive approach for regenerative medicine. Here, we show that 3,5-disubstituted isoxazoles (Isx), stem cell-modulator small molecules originally recovered in a P19 embryonal carcinoma cell-based screen, directed cardiac muscle gene expression in vivo in target tissues of adult transgenic reporter mice. Isx also stimulated adult mouse myocardial cell cycle activity. Narrowing our focus onto one target cardiac-resident progenitor population, Isx directed muscle transcriptional programs in vivo in multipotent Notch-activated epicardium-derived cells (NECs), generating Notch-activated adult cardiomyocyte-like precursors. Myocardial infarction (MI) preemptively differentiated NECs toward fibroblast lineages, overriding Isx's cardiogenic influence in this cell population. Isx dysregulated gene expression in vivo in Notch-activated repair fibroblasts, driving distinctive (pro-angiogenesis) gene programs, but failed to mitigate fibrosis or avert ventricular functional decline after MI. In NECs in vitro, Isx directed partial muscle differentiation, which included biosynthesis and assembly of sarcomeric α-actinin premyofibrils, beaded structures pathognomonic of early developing cardiomyocytes. Thus, although Isx small molecules have promising in vivo efficacy at the level of cardiac muscle gene expression in native multipotent progenitors and are first in class in this regard, a greater understanding of the dynamic interplay between fibrosis and cardiogenic small molecule signals will be required to pharmacologically enable regenerative repair of the heart.
Differentiation; research in biological diversity 2011
Small-molecule blocks malignant astrocyte proliferation and induces neuronal gene expression.
Abstract
Abstract
In the central nervous system (CNS), neural stem cells (NSCs) differentiate into neurons, astrocytes, and oligodendrocytes--these cell lineages are considered unidirectional and irreversible under normal conditions. The introduction of a defined set of transcription factors has been shown to directly convert terminally differentiated cells into pluripotent stem cells, reinforcing the notion that preserving cellular identity is an active process. Indeed, recent studies highlight that tumor suppressor genes (TSGs) such as Ink4a/Arf and p53, control the barrier to efficient reprogramming, leaving open the question whether the same TSGs function to maintain the differentiated state. During malignancy or following brain injury, mature astrocytes have been reported to re-express neuronal genes and re-gain neurogenic potential to a certain degree, yet few studies have addressed the underlying mechanisms due to a limited number of cellular models or tools to probe this process. Here, we use a synthetic small-molecule (isoxazole) to demonstrate that highly malignant EGFRvIII-expressing Ink4a/Arf(-/-); Pten(-/-) astrocytes downregulated their astrocyte character, re-entered the cell cycle, and upregulated neuronal gene expression. As a collateral discovery, isoxazole small-molecules blocked tumor cell proliferation in vitro, a phenotype likely coupled to activation of neuronal gene expression. Similarly, histone deacetylase inhibitors induced neuronal gene expression and morphologic changes associated with the neuronal phenotype, suggesting the involvement of epigenetic-mediated gene activation. Our study assesses the contribution of specific genetic pathways underlying the de-differentiation potential of astrocytes and uncovers a novel pharmacological tool to explore astrocyte plasticity, which may bring insight to reprogramming and anti-tumor strategies.
Proceedings of the National Academy of Sciences of the United States of America 2011
A small molecule differentiation inducer increases insulin production by pancreatic β cells.
Abstract
Abstract
New drugs for preserving and restoring pancreatic β-cell function are critically needed for the worldwide epidemic of type 2 diabetes and the cure for type 1 diabetes. We previously identified a family of neurogenic 3,5-disubstituted isoxazoles (Isx) that increased expression of neurogenic differentiation 1 (NeuroD1, also known as BETA2); this transcription factor functions in neuronal and pancreatic β-cell differentiation and is essential for insulin gene transcription. Here, we probed effects of Isx on human cadaveric islets and MIN6 pancreatic β cells. Isx increased the expression and secretion of insulin in islets that made little insulin after prolonged ex vivo culture and increased expression of neurogenic differentiation 1 and other regulators of islet differentiation and insulin gene transcription. Within the first few hours of exposure, Isx caused biphasic activation of ERK1/2 and increased bulk histone acetylation. Although there was little effect on histone deacetylase activity, Isx increased histone acetyl transferase activity in nuclear extracts. Reconstitution assays indicated that Isx increased the activity of the histone acetyl transferase p300 through an ERK1/2-dependent mechanism. In summary, we have identified a small molecule with antidiabetic activity, providing a tool for exploring islet function and a possible lead for therapeutic intervention in diabetes.
Nature chemical biology 2008
Small-molecule activation of neuronal cell fate.
Abstract
Abstract
We probed an epigenetic regulatory path from small molecule to neuronal gene activation. Isoxazole small molecules triggered robust neuronal differentiation in adult neural stem cells, rapidly signaling to the neuronal genome via Ca(2+) influx. Ca(2+)-activated CaMK phosphorylated and mediated nuclear export of the MEF2 regulator HDAC5, thereby de-repressing neuronal genes. These results provide new tools to explore the epigenetic signaling circuitry specifying neuronal cell fate and new leads for neuro-regenerative drugs.