IQ-1
WNT pathway activator; Inhibits protein phosphatase PP2A
概要
IQ-1 selectively inhibits p300-dependent β-catenin signaling by binding to the PR72/130 subunit of protein phosphatase PP2A, leading to decreased phosphorylation of the β-catenin coactivator, p300, and decreased affinity of p300 for β-catenin. IQ-1 thereby inhibits β-catenin/p300 interaction while increasing β-catenin/CBP mediated transcription (Miyabayashi et al.).
MAINTENANCE AND SELF-RENEWAL
· Used with Wnt3a to maintain pluripotency of mouse embryonic stem (ES) cells in the absence of mouse embryonic fibroblasts (MEFs), serum, or exogenous leukemia inhibitory factor (LIF; Miyabayashi et al.).
· Enhances expansion of mouse ES-derived cardiovascular progenitor cells (Schenke-Layland et al.).
CANCER RESEARCH
· Induces the conversion of cancer cells to a side population of cancer stem-like cells with high levels of drug resistance and tumorigenicity (He et al.).
MAINTENANCE AND SELF-RENEWAL
· Used with Wnt3a to maintain pluripotency of mouse embryonic stem (ES) cells in the absence of mouse embryonic fibroblasts (MEFs), serum, or exogenous leukemia inhibitory factor (LIF; Miyabayashi et al.).
· Enhances expansion of mouse ES-derived cardiovascular progenitor cells (Schenke-Layland et al.).
CANCER RESEARCH
· Induces the conversion of cancer cells to a side population of cancer stem-like cells with high levels of drug resistance and tumorigenicity (He et al.).
Alternative Names
Not applicable
Cell Type
Cancer Cells and Cell Lines, Cardiomyocytes, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Expansion, Maintenance
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
331001-62-8
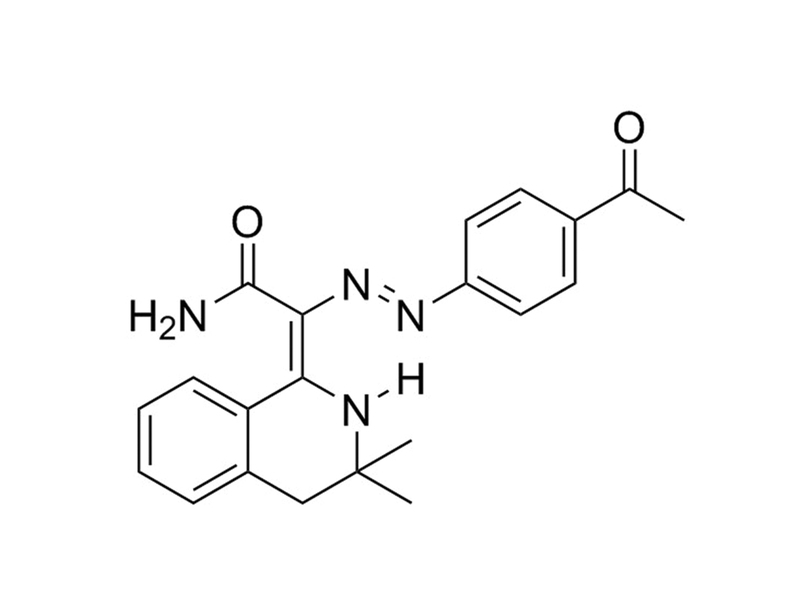
Chemical Formula
C₂₁H₂₂N₄O₂
Molecular Weight
362.4 g/mol
Purity
≥ 98%
Pathway
WNT
Target
PP2A
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | IQ-1 | 72772, 72774 | All | English |
| Safety Data Sheet | IQ-1 | 72772, 72774 | All | English |
数据及文献
Publications (3)
International journal of cancer 2014 JAN
Cancer cells acquire a drug resistant, highly tumorigenic, cancer stem-like phenotype through modulation of the PI3K/Akt/β-catenin/CBP pathway.
Abstract
Abstract
Cancer initiation and progression have been attributed to newly discovered subpopulations of self-renewing, highly tumorigenic, drug-resistant tumor cells termed cancer stem cells. Recently, we and others reported a new phenotypic plasticity wherein highly tumorigenic, drug-resistant cell populations could arise not only from pre-existing cancer stem-like populations but also from cancer cells lacking these properties. In the current study, we hypothesized that this newfound phenotypic plasticity may be mediated by PI3K/Akt and Wnt/β-catenin signaling, pathways previously implicated in carcinogenesis, pluripotency and drug resistance. Using GFP expression, Hoechst dye exclusion and fluorescence activated cell sorting (FACS) of cancer cell lines, we identified and tracked cancer stem-like side populations (SP) of cancer cells characterized by high tumorigenicity and drug resistance. We found that pharmacological inhibition or genetic depletion of PI3K and AKT markedly reduced the spontaneous conversion of nonside population (NSP) cells into cancer stem-like SP cells, whereas PI3K/Akt activation conversely enhanced NSP to SP conversion. PI3K/AKT signaling was mediated through downstream phosphorylation of GSK3β, which led to activation and accumulation of β-catenin. Accordingly, pharmacological or genetic perturbation of GSK3β or β-catenin dramatically impacted conversion of NSP to SP. Further downstream, β-catenin's effects on NSP-SP equilibrium were dependent upon its interaction with CBP, a KAT3 family coactivator. These studies provide a mechanistic model wherein PI3K/Akt/β-catenin/CBP signaling mediates phenotypic plasticity in and out of a drug-resistant, highly tumorigenic state. Therefore, targeting this pathway has unique potential for overcoming the therapy resistance and disease progression attributed to the cancer stem-like phenotype.
Biomaterials 2011 APR
Recapitulation of the embryonic cardiovascular progenitor cell niche.
Abstract
Abstract
Stem or progenitor cell populations are often established in unique niche microenvironments that regulate cell fate decisions. Although niches have been shown to be critical for the normal development of several tissues, their role in the cardiovascular system is poorly understood. In this study, we characterized the cardiovascular progenitor cell (CPC) niche in developing human and mouse hearts, identifying signaling pathways and extracellular matrix (ECM) proteins that are crucial for CPC maintenance and expansion. We demonstrate that collagen IV (ColIV) and β-catenin-dependent signaling are essential for maintaining and expanding undifferentiated CPCs. Since niches are three-dimensional (3D) structures, we investigated the impact of a 3D microenvironment that mimics the in vivo niche ECM. Employing electrospinning technologies, 3D in vitro niche substrates were bioengineered to serve as culture inserts. The three-dimensionality of these structures increased mouse embryonic stem cell differentiation into CPCs when compared to 2D control cultures, which was further enhanced by incorporation of ColIV into the substrates. Inhibiting p300-dependent β-catenin signals with the small molecule IQ1 facilitated further expansion of CPCs. Our study represents an innovative approach to bioengineer cardiac niches that can serve as unique 3D in vitro systems to facilitate CPC expansion and study CPC biology.
Proceedings of the National Academy of Sciences of the United States of America 2007 MAR
Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency.
Abstract
Abstract
Embryonic stem cells (ESCs) represent an important research tool and a potential resource for regenerative medicine. Generally, ESCs are cocultured with a supportive feeder cell layer of murine embryonic fibroblasts, which maintain the ESCs' capacity for self-renewal and block spontaneous differentiation. These cumbersome conditions, as well as the risk of xenobiotic contamination of human ESCs grown on murine embryonic fibroblasts, make it a priority to develop chemically defined methods that can be safely used for the expansion of ESCs. Using a high-throughput, cell-based assay, we identified the small molecule IQ-1 that allows for the Wnt/beta-catenin-driven long-term expansion of mouse ESCs and prevents spontaneous differentiation. We demonstrate that IQ-1, by targeting the PR72/130 subunit of the serine/threonine phosphatase PP2A, prevents beta-catenin from switching coactivator usage from CBP to p300. The increase in beta-catenin/CBP-mediated transcription at the expense of beta-catenin/p300-mediated transcription is critical for the maintenance of murine stem cell pluripotency.