GlyH-101
Inhibits cystic fibrosis transmembrane conductance regulator (CFTR)
概要
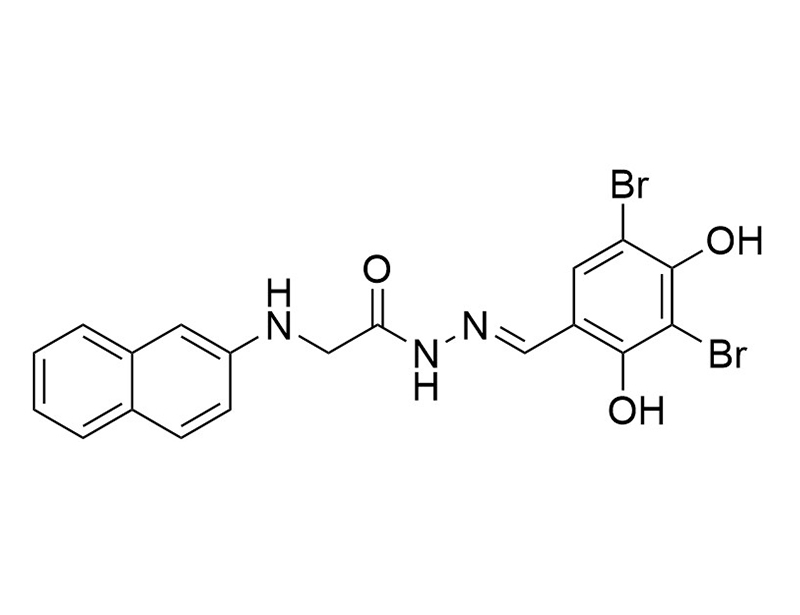
GlyH-101 is a cell-permeable glycine hydrazide that selectively and reversibly blocks the cystic fibrosis transmembrane conductance regulator (CFTR) channel (Ki = 4.3 µM; Sonawane et al.). The CFTR protein is a chloride anion channel involved in the secretion of fluid in many epithelial tissues, such as the airways and intestine (Ma et al.). Defects in the CFTR gene alter ion transport which can lead to cystic fibrosis (Dalli et al.; Ma et al.).
CANCER RESEARCH
· Blocks CFTR and inhibits cell division by inducing hyperpolarization in human gastric cancer cells (Zhu et al.).
CANCER RESEARCH
· Blocks CFTR and inhibits cell division by inducing hyperpolarization in human gastric cancer cells (Zhu et al.).
Alternative Names
CFTR inhibitor II
Cell Type
Airway Cells, Intestinal Cells
Area of Interest
Cancer Research, Epithelial Cell Biology
CAS Number
328541-79-3
Chemical Formula
C19H15Br2N3O3
Molecular Weight
493.2 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | GlyH-101 | 100-0530, 100-0531 | All | English |
| Safety Data Sheet | GlyH-101 | 100-0530, 100-0531 | All | English |
数据及文献
Publications (4)
Scientific reports 2018
Involvement of AMP-activated Protein Kinase (AMPK) in Regulation of Cell Membrane Potential in a Gastric Cancer Cell Line.
Abstract
Abstract
Membrane potential (Vmem) is a key bioelectric property of non-excitable cells that plays important roles in regulating cell proliferation. However, the regulation of Vmem itself remains largely unexplored. We found that, under nutrient starvation, during which cell division is inhibited, MKN45 gastric cancer cells were in a hyperpolarized state associated with a high intracellular chloride concentration. AMP-activated protein kinase (AMPK) activity increased, and expression of cystic fibrosis transmembrane conductance regulator (CFTR) decreased, in nutrient-starved cells. Furthermore, the increase in intracellular chloride concentration level and Vmem hyperpolarization in nutrient-starved cells was suppressed by inhibition of AMPK activity. Intracellular chloride concentrations and hyperpolarization increased after over-activation of AMPK using the specific activator AICAR or suppression of CFTR activity using specific inhibitor GlyH-101. Under these conditions, proliferation of MKN45 cells was inhibited. These results reveal that AMPK controls the dynamic change in Vmem by regulating CFTR and influencing the intracellular chloride concentration, which in turn influences cell-cycle progression. These findings offer new insights into the mechanisms underlying cell-cycle arrest regulated by AMPK and CFTR.
The American journal of pathology 2010 jul
CFTR inhibition provokes an inflammatory response associated with an imbalance of the annexin A1 pathway.
Abstract
Abstract
Cystic fibrosis (CF), a disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, is characterized by chronic bacterial infections and inflammation in the lung. Having previously shown that deletion of CFTR is associated with lower expression of the endogenous anti-inflammatory protein Annexin A1 (AnxA1), we investigated further this possible functional connection using a validated CFTR inhibitor. Treatment of mice with the CFTR inhibitor-172 (CFTR(172)) augmented the acute peritonitis promoted by zymosan, an effect associated with lower AnxA1 levels in peritoneal cells. Similar results were obtained with another, chemically distinct, CFTR inhibitor. The pro-inflammatory effect of CFTR(172) was lost in AnxA1(-/-), as well as CFTR(-/-) mice. Importantly, administration of hrAnxA1 and its peptido-mimetic to CFTR(-/-) animals or to animals treated with CFTR(172) corrected the exaggerated leukocyte migration seen in these animals. In vitro assays with human Polymorphonuclear leukocyte (PMN) demonstrated that CFTR(172) reduced cell-associated AnxA1 by promoting release of the protein in microparticles. We propose that the reduced impact of the counterregulatory properties of AnxA1 in CF cells contributes to the inflammatory phenotype characteristic of this disease. Thus, these findings provide an important insight into the mechanism underlying the inflammatory disease associated with CFTR inhibition while, at the same time, providing a novel pharmacological target for controlling the inflammatory phenotype of CF.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2006 jan
Luminally active, nonabsorbable CFTR inhibitors as potential therapy to reduce intestinal fluid loss in cholera.
Abstract
Abstract
Enterotoxin-mediated secretory diarrheas such as cholera involve chloride secretion by enterocytes into the intestinal lumen by the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. We previously identified glycine hydrazide CFTR blockers that by electrophysiological studies appeared to block the CFTR anion pore at its lumen-facing surface. Here, we synthesize highly water-soluble, nonabsorbable malondihydrazides by coupling 2,4-disulfobenzaldehyde, 4-sulfophenylisothiocyante, and polyethylene glycol (PEG) moieties to 2-naphthalenylamino-[(3,5-dibromo-2,4-dihydroxyphenyl) methylene] propanedioic acid dihydrazide, and aminoacethydrazides by coupling PEG to [(N-2-naphthalenyl)-2-(2-hydroxyethyl)]-glycine-2-[(3,5-dibromo-2,4-dihydroxyphenyl) methylene] hydrazide. Compounds rapidly, fully and reversibly blocked CFTR-mediated chloride current with Ki of 2-8 microM when added to the apical surface of epithelial cell monolayers. Compounds did not pass across Caco-2 monolayers, and were absorbed by {\textless}2{\%}/hr in mouse intestine. Luminally added compounds blocked by {\textgreater}90{\%} cholera toxin-induced fluid secretion in mouse intestinal loops, without inhibiting intestinal fluid absorption. These orally administered, nonabsorbable, nontoxic CFTR inhibitors may reduce intestinal fluid losses in cholera.
The Journal of clinical investigation 2002 dec
Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion.
Abstract
Abstract
Secretory diarrhea is the leading cause of infant death in developing countries and a major cause of morbidity in adults. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is required for fluid secretion in the intestine and airways and, when defective, causes the lethal genetic disease cystic fibrosis. We screened 50,000 chemically diverse compounds for inhibition of cAMP/flavone-stimulated Cl(-) transport in epithelial cells expressing CFTR. Six CFTR inhibitors of the 2-thioxo-4-thiazolidinone chemical class were identified. The most potent compound discovered by screening of structural analogs, CFTR(inh)-172, reversibly inhibited CFTR short-circuit current in less than 2 minutes in a voltage-independent manner with K(I) approximately 300 nM. CFTR(inh)-172 was nontoxic at high concentrations in cell culture and mouse models. At concentrations fully inhibiting CFTR, CFTR(inh)-172 did not prevent elevation of cellular cAMP or inhibit non-CFTR Cl(-) channels, multidrug resistance protein-1 (MDR-1), ATP-sensitive K(+) channels, or a series of other transporters. A single intraperitoneal injection of CFTR(inh)-172 (250 micro g/kg) in mice reduced by more than 90{\%} cholera toxin-induced fluid secretion in the small intestine over 6 hours. Thiazolidinone CFTR inhibitors may be useful in developing large-animal models of cystic fibrosis and in reducing intestinal fluid loss in cholera and other secretory diarrheas.