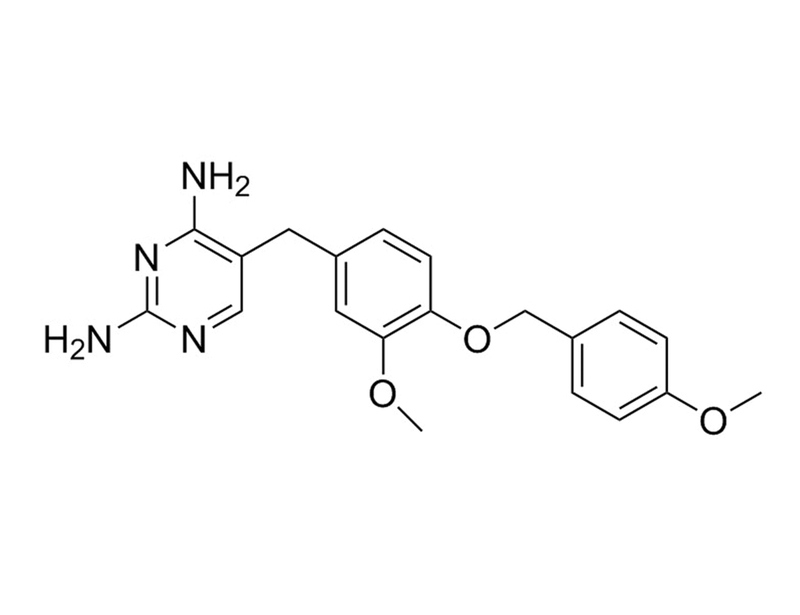
GW2580
CSF-1 pathway inhibitor; Inhibits cFMS
概要
GW2580 is a selective inhibitor of cFMS kinase (IC₅₀ = 0.03 μM) (Conway et al., 2008), blocking its ability to autophosphorylate colony-stimulating factor (CSF-1 or M-CSF), which promotes the survival, proliferation, and differentiation of macrophages.
DIFFERENTIATION
· Demonstrates the importance of CSF-1 in promoting myeloid lineage bias in mouse hematopoietic stem cells (Mossadegh-Keller et al.).
· Demonstrates the importance of CSF-1 in expansion of mouse and human macrophage colonies and monocytes (He et al.; Clanchy et al.; Conway et al., 2008; Conway et al., 2005).
· Inhibits bone degradation in cultures of human osteoclasts, rat calvaria, and rat fetal long bone (Conway et al., 2005), and in mouse models of arthritis (Conway et al., 2008).
DIFFERENTIATION
· Demonstrates the importance of CSF-1 in promoting myeloid lineage bias in mouse hematopoietic stem cells (Mossadegh-Keller et al.).
· Demonstrates the importance of CSF-1 in expansion of mouse and human macrophage colonies and monocytes (He et al.; Clanchy et al.; Conway et al., 2008; Conway et al., 2005).
· Inhibits bone degradation in cultures of human osteoclasts, rat calvaria, and rat fetal long bone (Conway et al., 2005), and in mouse models of arthritis (Conway et al., 2008).
Alternative Names
Not applicable
Cell Type
Mesenchymal Stem and Progenitor Cells, Myeloid Cells, Osteoblasts
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Disease Modeling, Stem Cell Biology
CAS Number
870483-87-7
Chemical Formula
C₂₀H₂₂N₄O₃
Molecular Weight
366.4 g/mol
Purity
≥ 98%
Pathway
CSF-1
Target
cFMS
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | GW2580 | 72472, 72474 | All | English |
| Safety Data Sheet | GW2580 | 72472, 72474 | All | English |
数据及文献
Publications (5)
Nature 2013 MAY
M-CSF instructs myeloid lineage fate in single haematopoietic stem cells.
Abstract
Abstract
Under stress conditions such as infection or inflammation the body rapidly needs to generate new blood cells that are adapted to the challenge. Haematopoietic cytokines are known to increase output of specific mature cells by affecting survival, expansion and differentiation of lineage-committed progenitors, but it has been debated whether long-term haematopoietic stem cells (HSCs) are susceptible to direct lineage-specifying effects of cytokines. Although genetic changes in transcription factor balance can sensitize HSCs to cytokine instruction, the initiation of HSC commitment is generally thought to be triggered by stochastic fluctuation in cell-intrinsic regulators such as lineage-specific transcription factors, leaving cytokines to ensure survival and proliferation of the progeny cells. Here we show that macrophage colony-stimulating factor (M-CSF, also called CSF1), a myeloid cytokine released during infection and inflammation, can directly induce the myeloid master regulator PU.1 and instruct myeloid cell-fate change in mouse HSCs, independently of selective survival or proliferation. Video imaging and single-cell gene expression analysis revealed that stimulation of highly purified HSCs with M-CSF in culture resulted in activation of the PU.1 promoter and an increased number of PU.1(+) cells with myeloid gene signature and differentiation potential. In vivo, high systemic levels of M-CSF directly stimulated M-CSF-receptor-dependent activation of endogenous PU.1 protein in single HSCs and induced a PU.1-dependent myeloid differentiation preference. Our data demonstrate that lineage-specific cytokines can act directly on HSCs in vitro and in vivo to instruct a change of cell identity. This fundamentally changes the current view of how HSCs respond to environmental challenge and implicates stress-induced cytokines as direct instructors of HSC fate.
Blood 2012 OCT
Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages.
Abstract
Abstract
Endothelial cells and macrophages are known to engage in tight and specific interactions that contribute to the modulation of vascular function. Here we show that adult endothelial cells provide critical signals for the selective growth and differentiation of macrophages from several hematopoietic progenitors. The process features the formation of well-organized colonies that exhibit progressive differentiation from the center to the periphery and toward an M2-like phenotype, characterized by enhanced expression of Tie2 and CD206/Mrc1. These colonies are long-lived depending on the contact with the endothelium; removal of the endothelial monolayer results in rapid colony dissolution. We further found that Csf1 produced by the endothelium is critical for the expansion of the macrophage colonies and that blockade of Csf1 receptor impairs colony growth. Functional analyses indicate that these macrophages are capable of accelerating angiogenesis, promoting tumor growth, and effectively engaging in tight associations with endothelial cells in vivo. These findings uncover a critical role of endothelial cells in the induction of macrophage differentiation and their ability to promote further polarization toward a proangiogenic phenotype. This work also highlights some of the molecules underlying the M2-like differentiation, a process that is relevant to the progression of both developmental and pathologic angiogenesis.
Cytokine 2012 JUL
HUVEC co-culture and haematopoietic growth factors modulate human proliferative monocyte activity.
Abstract
Abstract
Monocytes and macrophages are often claimed to have limited potential for proliferation in vivo and in vitro although a human monocyte subset with increased potential to proliferate in culture, termed the proliferative monocyte (PM), has previously been identified. The response of the putatively less mature PM to conditions conducive to haematopoietic stem cell culture was determined. Co-culture of monocytes on a HUVEC monolayer induced up to four cell divisions in a 9 day period. The PM response to haematopoietic growth factors (Flt3L, SCF, IL-6, IL-3 and M-CSF) was determined. M-CSF induced the greatest proliferative response in PM; IL-3 and Flt3L reduced basal and M-CSF-induced proliferation. The inhibition of M-CSFR kinase activity by GW2580 indicated that the ligand(s) for this receptor was a potent inducer of proliferation of this subset; inhibitors of intracellular signalling pathways also reduced PM proliferation.
The Journal of pharmacology and experimental therapeutics 2008 JUL
Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) in normal and arthritic rats.
Abstract
Abstract
The cFMS (cellular homolog of the V-FMS oncogene product of the Susan McDonough strain of feline sarcoma virus) (Proc Natl Acad Sci U S A 83:3331-3335, 1986) kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) inhibits colony-stimulating factor (CSF)-1-induced monocyte growth and bone degradation in vitro and inhibits CSF-1 signaling through cFMS kinase in 4-day models in mice (Proc Natl Acad Sci U S A 102:16078, 2005). In the present study, the kinase selectivity of GW2580 was further characterized, and the effects of chronic treatment were evaluated in normal and arthritic rats. GW2580 selectively inhibited cFMS kinase compared with 186 other kinases in vitro and completely inhibited CSF-1-induced growth of rat monocytes, with an IC(50) value of 0.2 microM. GW2580 dosed orally at 25 and 75 mg/kg 1 and 5 h before the injection of lipopolysaccharide inhibited tumor necrosis factor-alpha production by 60 to 85%, indicating a duration of action of at least 5 h. In a 21-day adjuvant arthritis model, GW2580 dosed twice a day (b.i.d.) from days 0 to 21, 7 to 21, or 14 to 21 inhibited joint connective tissue and bone destruction as assessed by radiology, histology and bone mineral content measurements. In contrast, GW2580 did not affect ankle swelling in the adjuvant model nor did it affect ankle swelling in a model where local arthritis is reactivated by peptidoglycan polysaccharide polymers. GW2580 administered to normal rats for 21 days showed no effects on tissue histology and only modest changes in serum clinical chemistry and blood hematology. In conclusion, GW2580 was effective in preserving joint integrity in the adjuvant arthritis model while showing minimal effects in normal rats.
Proceedings of the National Academy of Sciences of the United States of America 2005 NOV
Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580.
Abstract
Abstract
Colony-stimulating-factor-1 (CSF-1) signaling through cFMS receptor kinase is increased in several diseases. To help investigate the role of cFMS kinase in disease, we identified GW2580, an orally bioavailable inhibitor of cFMS kinase. GW2580 completely inhibited human cFMS kinase in vitro at 0.06 microM and was inactive against 26 other kinases. GW2580 at 1 microM completely inhibited CSF-1-induced growth of mouse M-NFS-60 myeloid cells and human monocytes and completely inhibited bone degradation in cultures of human osteoclasts, rat calvaria, and rat fetal long bone. In contrast, GW2580 did not affect the growth of mouse NS0 lymphoblastoid cells, human endothelial cells, human fibroblasts, or five human tumor cell lines. GW2580 also did not affect lipopolysaccharide (LPS)-induced TNF, IL-6, and prostaglandin E2 production in freshly isolated human monocytes and mouse macrophages. After oral administration, GW2580 blocked the ability of exogenous CSF-1 to increase LPS-induced IL-6 production in mice, inhibited the growth of CSF-1-dependent M-NFS-60 tumor cells in the peritoneal cavity, and diminished the accumulation of macrophages in the peritoneal cavity after thioglycolate injection. Unexpectedly, GW2580 inhibited LPS-induced TNF production in mice, in contrast to effects on monocytes and macrophages in vitro. In conclusion, GW2580's selective inhibition of monocyte growth and bone degradation is consistent with cFMS kinase inhibition. The ability of GW2580 to chronically inhibit CSF-1 signaling through cFMS kinase in normal and tumor cells in vivo makes GW2580 a useful tool in assessing the role of cFMS kinase in normal and disease processes.