Fumonisin B1
Inhibitor of sphingolipid synthesis and protein serine/threonine phosphatases
概要
Fumonisin B1 is a mycotoxin produced by Fusarium moniliforme that has been shown to potently inhibit sphingosine N-acyltransferase (ceramide synthase; Wang et al.), thereby disrupting the synthesis of sphingolipids, a key component of plasma membranes (IC₅₀ = 0.1 µM). Fumonisin B1 also inhibits protein serine/threonine phosphatases (PPs; PP1, PP2A, PP2B, PP2C, and PP5/T/K/H) with IC₅₀ values of 80 - 3000 μM. PP5 is the most sensitive with an IC₅₀ of 80 μM (Fukuda et al.). Fumonisin B1, together with Alfatoxin B1, increases reactive oxygen species levels and oxidative damage in rat spleen cells (Mary et al.).
MAINTENANCE & SELF-RENEWAL
· Reversibly blocks cell proliferation and DNA synthesis in Swiss 3T3 cells (Meivar-Levy et al.).
· Blocks hexadecylphosphocholine (HePC)-induced apoptosis in human keratinocyte cell lines (Wieder et al.).
DIFFERENTIATION
· Disrupts dendrite growth in cerebellar Purkinje neurons (Furuya et al).
· Inhibits axonal branching in cultured hippocampal neurons (Schwarz et al.).
CANCER RESEARCH
· Attenuates the response of mouse lymphoma cell lines to platelet-activating factor and blocks HePC-induced apoptosis by inhibiting ceramide formation (Balsinde et al.).
MAINTENANCE & SELF-RENEWAL
· Reversibly blocks cell proliferation and DNA synthesis in Swiss 3T3 cells (Meivar-Levy et al.).
· Blocks hexadecylphosphocholine (HePC)-induced apoptosis in human keratinocyte cell lines (Wieder et al.).
DIFFERENTIATION
· Disrupts dendrite growth in cerebellar Purkinje neurons (Furuya et al).
· Inhibits axonal branching in cultured hippocampal neurons (Schwarz et al.).
CANCER RESEARCH
· Attenuates the response of mouse lymphoma cell lines to platelet-activating factor and blocks HePC-induced apoptosis by inhibiting ceramide formation (Balsinde et al.).
Alternative Names
Not applicable
Cell Type
Cancer Cells and Cell Lines, Keratinocytes, Leukemia/Lymphoma Cells, Neurons
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Cancer Research, Neuroscience
CAS Number
116355-83-0
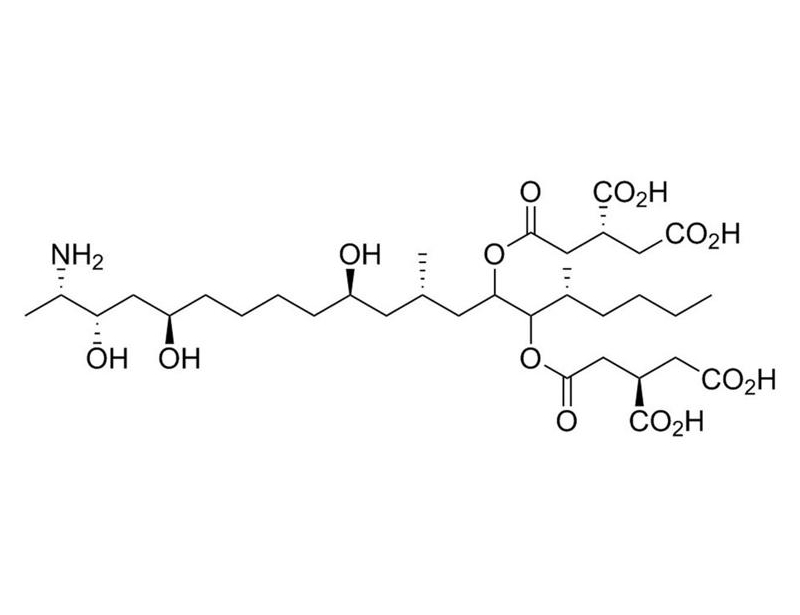
Chemical Formula
C₃₄H₅₉NO₁₅
Molecular Weight
721.8 g/mol
Purity
≥ 95%
Target
Sphingosine N-Acyltransferase
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Fumonisin B1 | 73682, 73684 | All | English |
| Safety Data Sheet | Fumonisin B1 | 73682, 73684 | All | English |
数据及文献
Publications (8)
Toxicology 2012 DEC
Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells.
Abstract
Abstract
Aflatoxin B1 (AFB(1)) and fumonisin B1 (FB(1)) are mycotoxins widely found as cereal contaminants. Their immunotoxicities predispose to infectious diseases and may alter the tumor immunosurveillance of human and animals, but the mechanisms underlying have not been fully elucidated, and the induction of oxidative stress has been proposed as a probable mechanism. This work was aimed at evaluating in spleen mononuclear cells (SMC) from Wistar rats the effects of the exposure, in vitro for up to 48 h, to 20 μM AFB(1), 10 μM FB(1) and AFB(1)-FB(1) mixture (MIX), over cellular oxidative status, as well as at elucidating the contribution of different reactive oxygen species (ROS) to biomolecular oxidative damage, the biochemical pathways involved, and the probable interaction of both toxins to induce oxidative stress. All the treatments increased total ROS and oxidation of biomolecules, with MIX having the greatest effects. However, only MIX increased superoxide anion radical. The main ROS involved in oxidation of proteins, lipids and DNA appear to be hydrogen peroxide and hydroxyl radical. The mitochondrial complex I and CYP450 were involved in the ROS generation induced by all treatments. The NADPH oxidase system was induced by FB1 and MIX. The arachidonic acid metabolism contributed to the ROS formation induced by AFB(1) and MIX. These results demonstrate that an interaction between AFB(1) and FB(1) occur in the oxidative stress induction, and show the biochemical pathways involved in ROS generation in SMC. The oxidative stress could mediate the AFB(1) and FB(1) individual and combined immunotoxicities.
The Journal of biological chemistry 1998 MAY
Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine.
Abstract
Abstract
The prototype of a new class of antiproliferative phospholipid analogs, hexadecylphosphocholine (HePC), has been shown to inhibit tumor growth and is currently used for the treatment of cutaneous metastases of mammary carcinomas. Although several cellular targets of HePC, e.g. protein kinase C and CTP:phosphocholine cytidylyltransferase, have been proposed, the mechanisms of HePC-induced anticancer activity are still unclear. Considering that the antiproliferative effect of HePC correlates with inhibition of phosphatidylcholine biosynthesis, which is tightly coupled to sphingomyelin biosynthesis, we tested the hypothesis that treatment of cells with the anticancer drug leads to increased cellular ceramide and subsequently to apoptotic cell death. In the present study, we showed that 25 micromol/liter HePC induced apoptosis. In further experiments, we demonstrated that HePC inhibited the incorporation of radiolabeled choline into phosphatidylcholine and at a later time point into sphingomyelin. This was confirmed by metabolic labeling of the lipid backbone using radiolabeled serine, and it was shown that HePC decreased the incorporation of serine into sphingomyelin by 35% and simultaneously increased the incorporation of serine into ceramide by 70%. Determination of the amount of ceramide revealed an increase of 53% in HePC-treated cells compared with controls. In accordance with the hypothesis that elevated ceramide levels may be the missing link between the metabolic effects of HePC and its proapoptotic properties, HePC-induced apoptosis was blocked by fumonisin B1, an inhibitor of ceramide synthesis. Furthermore, we found that membrane-permeable ceramides additively increased the apoptotic effect of HePC.
The Journal of biological chemistry 1997 JAN
The role of sphingolipids in the maintenance of fibroblast morphology. The inhibition of protrusional activity, cell spreading, and cytokinesis induced by fumonisin B1 can be reversed by ganglioside GM3.
Abstract
Abstract
Previous studies demonstrated that inhibition of sphingolipid synthesis by the mycotoxin fumonisin B1 (FB1) disrupts axonal growth in cultured hippocampal neurons (Harel, R., and Futerman, A. H. (1993) J. Biol. Chem. 268, 14476-14481) by affecting the formation or stabilization of axonal branches (Schwarz, A., Rapaport, E., Hirschberg, K., and Futerman, A.H. (1995) J. Biol. Chem. 270, 10990-10998). We now demonstrate that long term incubation with FB1 affects fibroblast morphology and proliferation. Incubation of Swiss 3T3 cells with FB1 resulted in a decrease in synthesis of ganglioside GM3, the major glycosphingolipid in 3T3 fibroblasts and of sphingomyelin. The projected cell area of FB1-treated cells was approximately 45% less than control cells. FB1 had no affect on the organization of microtubules or intermediate filaments, but fewer actin-rich stress fibers were observed, and there was a loss of actin-rich lamellipodia at the leading edge. Three other processes involving the actin cytoskeleton, cytokinesis, microvilli formation, and the formation of long processes induced by protein kinase inhibitors, were all disrupted by FB1. All the effects of FB1 on cell morphology could be reversed by addition of ganglioside GM3 even in the presence of FB1, whereas the bioactive intermediates, sphinganine, sphingosine, and ceramide, were without effect. Finally, FB1 blocked cell proliferation and DNA synthesis in a reversible manner, although ganglioside GM3 could not reverse the effects of FB1 on cell proliferation. Together, these data suggest that ongoing sphingolipid synthesis is required for the assembly of both new membrane and of the underlying cytoskeleton.
The Journal of biological chemistry 1997 AUG
Inflammatory activation of arachidonic acid signaling in murine P388D1 macrophages via sphingomyelin synthesis.
Abstract
Abstract
Ceramide has emerged as an important lipid messenger for many cellular processes triggered via surface receptors. In the present study, inflammatory activation of P388D1 macrophages with bacterial lipopolysaccharide (LPS) and platelet-activating factor (PAF) stimulated a transient accumulation of ceramide. Moreover, cell-permeable ceramide mimicked LPS/PAF in triggering arachidonate mobilization in these cells. LPS/PAF-induced ceramide synthesis did not result from sphingomyelinase activation but from increased de novo synthesis. Participation of this pathway in arachidonate signaling was detected since fumonisin B1, an inhibitor of de novo ceramide synthesis, was able to inhibit the LPS/PAF-induced response. These studies have uncovered a new role for sphingolipid metabolism in cellular signaling and constitute evidence that products of the sphingomyelin biosynthetic pathway may serve a specific role in signal transduction by influencing the activity of the novel Group V secretory phospholipase A2.
Biochemical and biophysical research communications 1996 MAR
Inhibition of protein serine/threonine phosphatases by fumonisin B1, a mycotoxin.
Abstract
Abstract
Fumonisin B1 (FB1), a mycotoxin produced by the fungus Fusarium moniliforme, which is a common contaminant of corn, is suspected to be a cause of human esophageal cancer. FB1 is hepatotoxic and hepatocarcinogenic in rats, and although the mechanisms involved have not been clarified, the latter is associated with a weak initiating activity. The effects of FB1 on the activity of protein serine/threonine phosphatases (PPs) (PP1, PP2A, PP2B, PP2C and PP5/T/K/H) were investigated in the present study. Inhibition of dephosphorylation was noted for all five PPs with IC50 values of 80 microM-3000 microM. Among the five PPs examined, PP5 was most sensitive with an IC50 of 80 microM. This concentration is comparable to that estimated to be reached in the rat body by feeding FB1 to obtain hepatic tumors. Inhibition of PP5 could thus play important roles in the toxicity and carcinogenic action of FB1.
Journal of neurochemistry 1995 OCT
Sphingolipid biosynthesis is necessary for dendrite growth and survival of cerebellar Purkinje cells in culture.
Abstract
Abstract
The requirement of complex sphingolipid biosynthesis for growth of neurons was examined in developing rat cerebellar Purkinje neurons using a dissociated culture system. Purkinje cells developed well-differentiated dendrites and axons after 2 weeks in a serum-free nutrient condition. Addition of 2 microM fumonisin B1, a fungal inhibitor of mammalian ceramide synthase, inhibited incorporation of [3H]galactose/glucosamine and [14C]-serine into complex sphingolipids of cultured cerebellar neurons. Under this condition, the expression of Purkinje cell-enriched sphingolipids, including GD1 alpha, 9-O-acetylated LD1 and GD3, and sphingomyelin, was significantly decreased. After 2 weeks' exposure to fumonisin B1, dose-dependent measurable decreases in the survival and visually discernible differences in the morphology were seen in fumonisin-treated Purkinje cells. The Purkinje cell dendrites exhibited two types of anomalies; one population of cells developed elongated but less-branched dendrites after a slight time lag, but their branches began to degenerate. In some cells, formation of elongated dendrite trees was severely impaired. However, treatment with fumonisin B1 also led to the formation of spinelike protrusions on the dendrites of Purkinje cells as in control cultures. In contrast to the alterations observed in Purkinje cells, morphology of other cell types including granule neurons appeared to be almost normal after treatment with fumonisin B1. These observations indicated strongly that membrane sphingolipids participate in growth and maintenance of dendrites and in the survival of cerebellar Purkinje cells. Indeed, these effects of fumonisin B1 were reversed, but not completely, by the addition of 6-[[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino dcaproyl]sphingosine (C6-NBD-ceramide), a synthetic derivative of ceramide. Thus, we conclude that deprivation of membrane sphingolipids in a culture environment is responsible for aberrant growth of Purkinje cells.