Fluoxetine
Serotonin reuptake inhibitor
概要
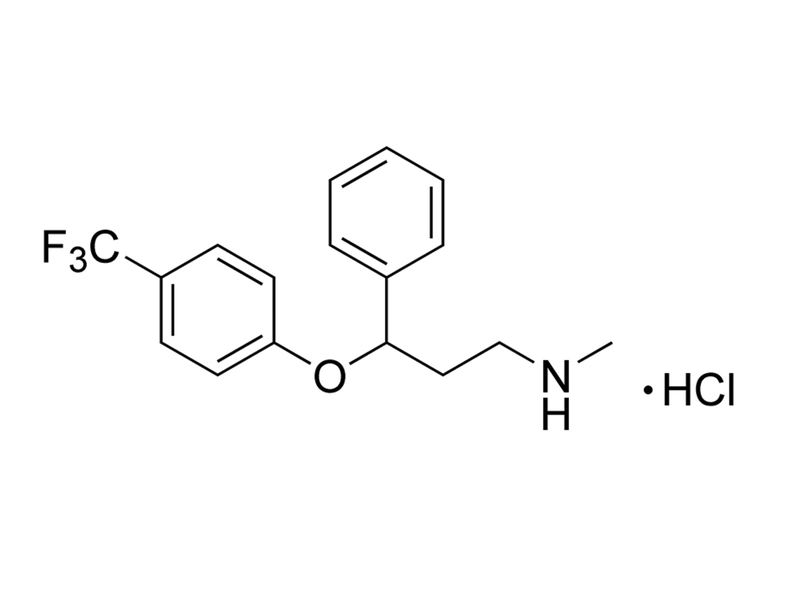
Fluoxetine is a selective serotonin reuptake inhibitor, displaying a distinct preference for the serotonin transporter (Kd = 0.81 nM) over both the norepinephrine and dopamine transporters (Kd values of 240 and 3600 nM, respectively; Tatsumi et al.). This product is supplied as a hydrochloride salt of the molecule.
DIFFERENTIATION
· Induces proliferation of human embryonic stem cell-derived neural precursors, and enhances their neuronal differentiation (Chang et al. 2010).
MAINTENANCE
· Induces proliferation and inhibits differentiation of human hypothalamic neuroprogenitor cells (Sousa-Ferreira et al.).
· Stimulates proliferation of mouse fetal neural stem cells (Chang et al. 2012).
· Stimulates in vivo proliferation of amplifying neural progenitor cells in adult mouse brain (Encinas et al.).
DIFFERENTIATION
· Induces proliferation of human embryonic stem cell-derived neural precursors, and enhances their neuronal differentiation (Chang et al. 2010).
MAINTENANCE
· Induces proliferation and inhibits differentiation of human hypothalamic neuroprogenitor cells (Sousa-Ferreira et al.).
· Stimulates proliferation of mouse fetal neural stem cells (Chang et al. 2012).
· Stimulates in vivo proliferation of amplifying neural progenitor cells in adult mouse brain (Encinas et al.).
Alternative Names
LY110140
Cell Type
Neural Cells, PSC-Derived, Neural Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Expansion, Maintenance
Area of Interest
Neuroscience, Stem Cell Biology
CAS Number
56296-78-7
Chemical Formula
C₁₇H₁₈F₃NO · HCl
Molecular Weight
345.8 g/mol
Purity
≥ 98%
Target
Serotonin Transporter
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Fluoxetine (Hydrochloride) | 73142, 73144 | All | English |
| Safety Data Sheet | Fluoxetine (Hydrochloride) | 73142, 73144 | All | English |
数据及文献
Publications (5)
PloS one 2014
Fluoxetine induces proliferation and inhibits differentiation of hypothalamic neuroprogenitor cells in vitro.
Abstract
Abstract
A significant number of children undergo maternal exposure to antidepressants and they often present low birth weight. Therefore, it is important to understand how selective serotonin reuptake inhibitors (SSRIs) affect the development of the hypothalamus, the key center for metabolism regulation. In this study we investigated the proliferative actions of fluoxetine in fetal hypothalamic neuroprogenitor cells and demonstrate that fluoxetine induces the proliferation of these cells, as shown by increased neurospheres size and number of proliferative cells (Ki-67+ cells). Moreover, fluoxetine inhibits the differentiation of hypothalamic neuroprogenitor cells, as demonstrated by decreased number of mature neurons (Neu-N+ cells) and increased number of undifferentiated cells (SOX-2+ cells). Additionally, fluoxetine-induced proliferation and maintenance of hypothalamic neuroprogenitor cells leads to changes in the mRNA levels of appetite regulator neuropeptides, including Neuropeptide Y (NPY) and Cocaine-and-Amphetamine-Regulated-Transcript (CART). This study provides the first evidence that SSRIs affect the development of hypothalamic neuroprogenitor cells in vitro with consequent alterations on appetite neuropeptides.
Neurochemistry international 2012
Therapeutic potentials of neural stem cells treated with fluoxetine in Alzheimer's disease.
Abstract
Abstract
Recent studies have proposed that chronic treatment with antidepressants increases neurogenesis in the adult hippocampus. However, the effect of antidepressants on fetal neural stem cells (NSCs) has not been well defined. Our study shows the dose-dependent effects of fluoxetine on the proliferation and neural differentiation of NSCs. Fluoxetine, even at nanomolar concentrations, stimulated proliferation of NSCs and increased the number of βIII-tubulin (Tuj 1)- and neural nucleus marker (NeuN)-positive cells, but not glial fibrillary acidic protein (GFAP)-positive cells. These results suggest that fluoxetine can enhance neuronal differentiation. In addition, fluoxetine has protective effects against cell death induced by oligomeric amyloid beta (Aβ(42)) peptides. Taken together, these results clearly show that fluoxetine promotes both the proliferation and neuronal differentiation of NSCs and exerts protective effects against Aβ(42)-induced cytotoxicities in NSCs, which suggest that the use of fluoxetine is applicable for cell therapy for various neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases by its actions in NSCs.
The International journal of developmental biology 2010
Increased cellular turnover in response to fluoxetine in neuronal precursors derived from human embryonic stem cells.
Abstract
Abstract
Previous reports have shown that antidepressants increase neuronal cell proliferation and enhance neuroplasticity both in vivo and in vitro. This study investigated the direct effects of one such antidepressant, fluoxetine , on cell proliferation and on the production of neurotrophic factors in neuronal precursors derived from human embryonic stem cells (hESCs; H9). Fluoxetine induced the differentiation of neuronal precursors, strongly enhancing neuronal characteristics. The rate of proliferation was higher in fluoxetine -treated cells than in control cells, as determined by MTT [3(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide] assay. The CPDL (cumulative population doubling level) of the fluoxetine-treated cells was significantly increased in comparison to that of control cells (ptextless.001). Bromodeoxyuridine incorporation and staurosporine-induced apoptosis assays were elevated in fluoxetine-treated cells. Quantitative RT-PCR analysis revealed no significant differences in the expression of neurotrophic factors, brain-derived neurotrophic factor (BDNF);glial-derived neurotrophic factor (GDNF) and cAMP-responsive element-binding protein (CREB) between cells treated with fluoxetine for two weeks and their untreated counterparts. These results may help elucidate the mechanism of action of fluoxetine as a therapeutic drug for the treatment of depression. Data presented herein provide more evidence that, in addition to having a direct chemical effect on serotonin levels, fluoxetine can influence hESC-derived neuronal cells by increasing cell proliferation, while allowing them to maintain their neuronal characteristics.
Proceedings of the National Academy of Sciences of the United States of America 2006 MAY
Fluoxetine targets early progenitor cells in the adult brain.
Abstract
Abstract
Chronic treatment with antidepressants increases neurogenesis in the adult hippocampus. This increase in the production of new neurons may be required for the behavioral effects of antidepressants. However, it is not known which class of cells within the neuronal differentiation cascade is targeted by the drugs. We have generated a reporter mouse line, which allows identification and classification of early neuronal progenitors. It also allows accurate quantitation of changes induced by neurogenic agents in these distinct subclasses of neuronal precursors. We use this line to demonstrate that the selective serotonin reuptake inhibitor antidepressant fluoxetine does not affect division of stem-like cells in the dentate gyrus but increases symmetric divisions of an early progenitor cell class. We further demonstrate that these cells are the sole class of neuronal progenitors targeted by fluoxetine in the adult brain and suggest that the fluoxetine-induced increase in new neurons arises as a result of the expansion of this cell class. This finding defines a cellular target for antidepressant drug therapies.
European journal of pharmacology 1997 DEC
Pharmacological profile of antidepressants and related compounds at human monoamine transporters.
Abstract
Abstract
Using radioligand binding assays, we determined the equilibrium dissociation constants (KD's) for 37 antidepressants, three of their metabolites (desmethylcitalopram, desmethylsertraline, and norfluoxetine), some mood stabilizers, and assorted other compounds (some antiepileptics, Ca2+ channel antagonists, benzodiazepines, psychostimulants, antihistamines, and monoamines) for the human serotonin, norepinephrine, and dopamine transporters. Among the compounds that we tested, mazindol was the most potent at the human norepinephrine and dopamine transporters with KD's of 0.45 +/- 0.03 nM and 8.1 +/- 0.4 nM, respectively. Sertraline (KD = 25 +/- 2 nM) and nomifensine (56 +/- 3 nM) were the two most potent antidepressants at the human dopamine transporter. We showed significant correlations for antidepressant affinities at binding to serotonin (R = 0.93), norepinephrine (R = 0.97), and dopamine (R = 0.87) transporters in comparison to their respective values for inhibiting uptake of monoamines into rat brain synaptosomes. These data are useful in predicting some possible adverse effects and drug-drug interactions of antidepressants and related compounds.