Everolimus
mTOR pathway inhibitor; Inhibits FKBP-12
概要
Everolimus is an inhibitor of the mammalian target of rapamycin (mTOR). It is a hydroxyethyl ether-substituted derivative of rapamycin with improved pharmacokinetic and pharmacodynamic properties. It inhibits both mTORC1 and mTORC2 complexes by binding to FK506-binding protein (FKBP-12), which then binds to mTOR, leading to complex destabilization and blocked kinase function (Zeng et al.; Lebwohl et al.; Sedrani et al.; Huang & Houghton).
CANCER RESEARCH
· Inhibits cell proliferation, metabolism, and angiogenesis in a variety of cancers using in vitro and in vivo models (Zhu et al.; Lane et al.; O’Reilly et al.; Lebwohl et al.).
IMMUNOLOGY
· Acts as an immunosuppressive agent in the context of organ transplantation (Lebwohl et al.; Wullschleger et al.).
CANCER RESEARCH
· Inhibits cell proliferation, metabolism, and angiogenesis in a variety of cancers using in vitro and in vivo models (Zhu et al.; Lane et al.; O’Reilly et al.; Lebwohl et al.).
IMMUNOLOGY
· Acts as an immunosuppressive agent in the context of organ transplantation (Lebwohl et al.; Wullschleger et al.).
Alternative Names
RAD001; SDZ-RAD; Xience
Cell Type
Cancer Cells and Cell Lines
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research, Immunology, Transplantation Research
CAS Number
159351-69-6
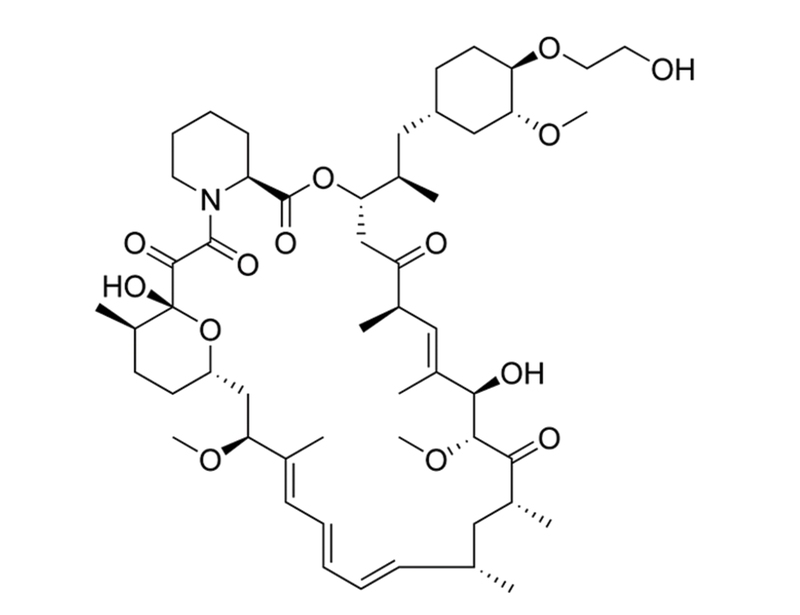
Chemical Formula
C₅₃H₈₃NO₁₄
Molecular Weight
958.2 g/mol
Purity
≥ 95%
Pathway
mTOR
Target
FKBP-12
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | Everolimus | 73122, 73124 | Purchase date 2019-09-12 or earlier | English |
| Product Information Sheet 2 | Everolimus | 73122, 73124 | Purchase date 2019-09-13 or later | English |
| Safety Data Sheet | Everolimus | 73122, 73124 | All | English |
数据及文献
Publications (7)
Annals of the New York Academy of Sciences 2013
Development of everolimus, a novel oral mTOR inhibitor, across a spectrum of diseases.
Abstract
Abstract
Everolimus is a potent, oral inhibitor of the mammalian target of rapamycin (mTOR) that has been investigated in multiple clinical development programs since 1996. A unique collaboration between academic and pharmaceutical experts fostered research that progressed rapidly, with simultaneous indication findings across numerous tumor types. Initially developed for the prophylaxis of organ transplant rejection, everolimus has demonstrated efficacy and safety for the treatment of patients with various types of cancer (renal cell carcinoma, neuroendocrine tumors of pancreatic origin, and breast cancer) and for adult and pediatric patients with tuberous sclerosis complex. The FDA approval of everolimus for these diseases has addressed several unmet medical needs and is widely accepted by the medical community where treatment options may be limited. An extensive clinical development program is ongoing to establish the role of everolimus as monotherapy, or in combination with other agents, in the treatment of a broad spectrum of malignancies.
Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2012
Antitumor effect of the mTOR inhibitor everolimus in combination with trastuzumab on human breast cancer stem cells in vitro and in vivo.
Abstract
Abstract
This study evaluated the effects of a mammalian target of mTOR inhibitor everolimus alone or in combination with trastuzumab on stem cells from HER2-overexpressing primary breast cancer cells and the BT474 breast cancer cell line in vitro and in vivo. For the in vitro studies, we sorted ESA(+)CD44(+)CD24(-/low) cells as stem cells from primary breast cancer cells and BT474 cells using flow cytometry. The MTT assay was used to quantify the inhibitory effect of the drugs on total cells and stem cells specifically. Stem cell apoptosis, cell cycle distributions, and their tumorigenicity after treatment were investigated by flow cytometry or soft agar colony formation assays. For the in vivo studies, BALB/c mice were injected with BT474 stem cells, and the different treatments were administered. After necropsy, the expression of Ki67, CD31, AKT1, and phospho-AKT (Thr308) was analyzed by immunohistochemistry. For the in vitro studies, Treatment with everolimus resulted in stem cell growth inhibition in a dose-dependent manner. The combination of everolimus with trastuzumab was more effective at inhibiting cell growth (P textless 0.001) and tumorigenicity (P textless 0.001) compared with single-agent therapy. In addition, an increase in G1 cell cycle arrest and an increased population of cells in early apoptosis were seen in the combination treatment group compared with either of the single-agent groups (P textless 0.01). For the in vivo studies, everolimus plus trastuzumab therapy was much more effective at reducing tumor volume in mice compared with either single agent alone (P textless 0.05). Compared with everolimus alone, the combination of everolimus and trastuzumab reduced the expression of Ki67, AKT1, and phospho-AKT (Thr308) (P textless 0.05). We conclude that everolimus has effective inhibitory effects on HER2-overexpressing stem cells in vitro and vivo. Everolimus plus trastuzumab is a rational combination treatment that may be promising in human clinical trials.
Clinical cancer research : an official journal of the American Association for Cancer Research 2009
mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor.
Abstract
Abstract
PURPOSE: Comparison of the antiangiogenic/vascular properties of the oral mammalian target of rapamycin (mTOR) inhibitor RAD001 (everolimus) and the vascular endothelial growth factor receptor (VEGFR) inhibitor vatalanib (PTK/ZK). EXPERIMENTAL DESIGN: Antiproliferative activity against various tumor histotypes and downstream effects on the mTOR pathway were measured in vitro. In vivo, antitumor activity, plasma, and tumor RAD001 levels were measured. Activity in several different angiogenic/vascular assays in vitro and in vivo was assessed and compared with PTK/ZK. RESULTS: RAD001 inhibited proliferation in vitro (IC50 valuestextless1 nmol/L to textgreater1 micromol/L), and in sensitive and insensitive tumor cells, pS6 kinase and 4E-BP1 were inhibited. Activity in vitro did not correlate with activity in vivo and significant responses were seen in tumors with IC50 valuestextgreater10-fold higher than tumor RAD001 concentrations. In vitro, RAD001 inhibited the proliferation of VEGF-stimulated and fibroblast growth factor-stimulated human endothelial cells but not dermal fibroblasts and impaired VEGF release from both sensitive and insensitive tumor cells but did not inhibit migration of human endothelial cells. In vivo, in tumor models derived from either sensitive or insensitive cells, RAD001 reduced Tie-2 levels, the amount of mature and immature vessels, total plasma, and tumor VEGF. RAD001 did not affect blood vessel leakiness in normal vasculature acutely exposed to VEGF nor did it affect tumor vascular permeability (Ktrans) as measured by dynamic contrast-enhanced magnetic resonance imaging. However, the pan-VEGFR inhibitor PTK/ZK inhibited endothelial cell migration and vascular permeability but had less effect on mature vessels compared with RAD001. CONCLUSIONS: VEGFR and mTOR inhibitors show similar but also distinct effects on tumor vascular biology, which has implications for their clinical activity alone or in combination.
Blood 2007
Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML.
Abstract
Abstract
The mTOR complex 2 (mTORC2) containing mTOR and rictor is thought to be rapamycin insensitive and was recently shown to regulate the prosurvival kinase AKT by phosphorylation on Ser473. We investigated the molecular effects of mTOR inhibition by the rapamycin derivatives (RDs) temsirolimus (CCI-779) and everolimus (RAD001) in acute myeloid leukemia (AML) cells. Unexpectedly, RDs not only inhibited the mTOR complex 1 (mTORC1) containing mTOR and raptor with decreased p70S6K, 4EPB1 phosphorylation, and GLUT1 mRNA, but also blocked AKT activation via inhibition of mTORC2 formation. This resulted in suppression of phosphorylation of the direct AKT substrate FKHR and decreased transcription of D-cyclins in AML cells. Similar observations were made in samples from patients with hematologic malignancies who received RDs in clinical studies. Our study provides the first evidence that rapamycin derivatives inhibit AKT signaling in primary AML cells both in vitro and in vivo, and supports the therapeutic potential of mTOR inhibition strategies in leukemias.
Cell 2006
TOR signaling in growth and metabolism.
Abstract
Abstract
The target of rapamycin (TOR) is a conserved Ser/Thr kinase that regulates cell growth and metabolism in response to environmental cues. Here, highlighting contributions from studies in model organisms, we review mammalian TOR complexes and the signaling branches they mediate. TOR is part of two distinct multiprotein complexes, TOR complex 1 (TORC1), which is sensitive to rapamycin, and TORC2, which is not. The physiological consequences of mammalian TORC1 dysregulation suggest that inhibitors of mammalian TOR may be useful in the treatment of cancer, cardiovascular disease, autoimmunity, and metabolic disorders.
Current opinion in pharmacology 2003 AUG
Targeting mTOR signaling for cancer therapy.
Abstract
Abstract
The mammalian target of rapamycin (mTOR), an atypical serine/threonine kinase, plays a central role in the regulation of cell proliferation, growth, differentiation, migration and survival. Dysregulation of mTOR signaling occurs in diverse human tumours, and can confer higher susceptibility to inhibitors of mTOR. Rapamycin and its derivatives, CCI-779 and RAD001 (designated rapamycins), specifically inhibit the function of mTOR, leading to inactivation of ribosomal S6K1 and inhibition of cap-dependent translation initiation through the 4E-BP1/eIF4E pathway. The overall effect is an accumulation of cells in the G1 phase of the cell-cycle, and potential apoptosis. Preclinical studies indicate that rapamycins are potent inhibitors of the proliferation of numerous tumour cell lines in culture and of murine syngeneic tumour models or human xenografts. RAD001 and CCI-779 are in phase I and II trials, respectively, as anti-cancer agents. These trials have demonstrated promising anti-cancer activity and relatively mild side effects of CCI-779. Emerging results suggest that inhibition of mTOR signaling can be exploited as a potential tumour-selective therapeutic strategy.