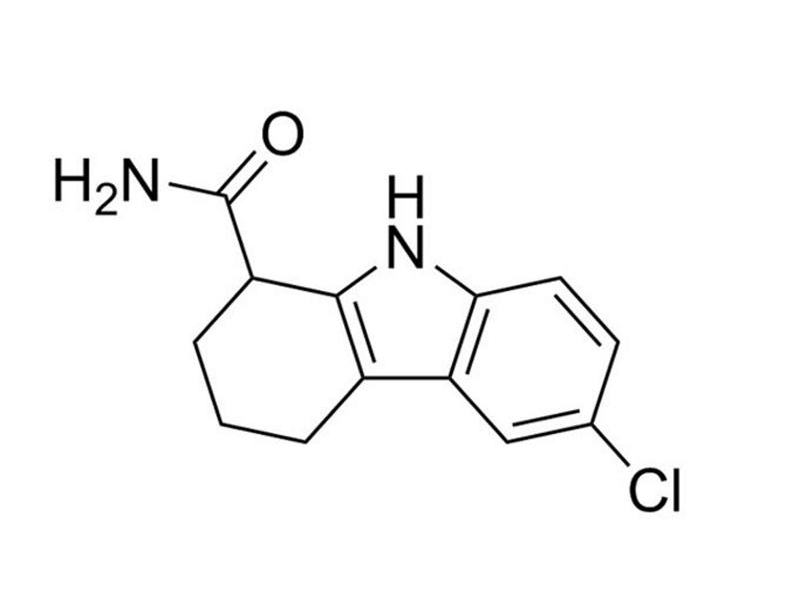
EX527
Epigenetic modifier; Inhibits SIRT1 histone deacetylase
概要
EX527 is a cell-permeable, selective inhibitor of mammalian sirtuin 1 (SIRT1; IC₅₀ = 98 nM) over SIRT2 and SIRT3 and has no effect on other histone deacetylases (HDACs; Nayagam et al.). SIRT1 is a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase with roles in energy metabolism and inflammation. Studies have shown that EX527 inhibits sirtuins by forming a trimeric sirtuin complex with an NAD+-derived coproduct (Gertz M et al.).
DIFFERENTIATION
· Increases the production of oligodendrocytes from differentiating neural stem cells and neural progenitor cells in vitro (Rafalski et al.).
IMMUNOLOGY
· Restores the microvascular response during the hypoinflammatory phase in a mouse model of sepsis, and enhances the systemic innate immune response (Vachharajani et al.).
DISEASE MODELING
· Delays cyst growth in kidneys of PKD1 knockout mouse models (Zhou et al.)
DIFFERENTIATION
· Increases the production of oligodendrocytes from differentiating neural stem cells and neural progenitor cells in vitro (Rafalski et al.).
IMMUNOLOGY
· Restores the microvascular response during the hypoinflammatory phase in a mouse model of sepsis, and enhances the systemic innate immune response (Vachharajani et al.).
DISEASE MODELING
· Delays cyst growth in kidneys of PKD1 knockout mouse models (Zhou et al.)
Alternative Names
Selisistat
Cell Type
Neural Stem and Progenitor Cells, Renal Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Disease Modeling, Immunology
CAS Number
49843-98-3
Chemical Formula
C₁₃H₁₃ClN₂O
Molecular Weight
248.7 g/mol
Purity
≥ 98%
Pathway
Epigenetic
Target
HDAC
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EX527 | 73654 | All | English |
| Safety Data Sheet | EX527 | 73654 | All | English |
数据及文献
Publications (5)
Journal of leukocyte biology 2014 NOV
SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome.
Abstract
Abstract
Mechanism-based sepsis treatments are unavailable, and their incidence is rising worldwide. Deaths occur during the early acute phase of hyperinflammation or subsequent postacute hypoinflammatory phase with sustained organ failure. The acute sepsis phase shifts rapidly, and multiple attempts to treat early excessive inflammation have uniformly failed. We reported in a sepsis cell model and human sepsis blood leukocytes that nuclear NAD+ sensor SIRT1 deacetylase remodels chromatin at specific gene sets to switch the acute-phase proinflammatory response to hypoinflammatory. Importantly, SIRT1 chromatin reprogramming is reversible, suggesting that inhibition of SIRT1 might reverse postacute-phase hypoinflammation. We tested this concept in septic mice, using the highly specific SIRT1 inhibitor EX-527, a small molecule that closes the NAD+ binding site of SIRT1. Strikingly, when administered 24 h after sepsis, all treated animals survived, whereas only 40% of untreated mice survived. EX-527 treatment reversed the inability of leukocytes to adhere at the small intestine MVI, reversed in vivo endotoxin tolerance, increased leukocyte accumulation in peritoneum, and improved peritoneal bacterial clearance. Mechanistically, the SIRT1 inhibitor restored repressed endothelial E-selectin and ICAM-1 expression and PSGL-1 expression on the neutrophils. Systemic benefits of EX-527 treatment included stabilized blood pressure, improved microvascular blood flow, and a shift toward proimmune macrophages in spleen and bone marrow. Our findings reveal that modifying the SIRT1 NAD+ axis may provide a novel way to treat sepsis in its hypoinflammatory phase.
Nature cell biology 2013 JUN
Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain.
Abstract
Abstract
Oligodendrocytes-the myelin-forming cells of the central nervous system-can be regenerated during adulthood. In adults, new oligodendrocytes originate from oligodendrocyte progenitor cells (OPCs), but also from neural stem cells (NSCs). Although several factors supporting oligodendrocyte production have been characterized, the mechanisms underlying the generation of adult oligodendrocytes are largely unknown. Here we show that genetic inactivation of SIRT1, a protein deacetylase implicated in energy metabolism, increases the production of new OPCs in the adult mouse brain, in part by acting in NSCs. New OPCs produced following SIRT1 inactivation differentiate normally, generating fully myelinating oligodendrocytes. Remarkably, SIRT1 inactivation ameliorates remyelination and delays paralysis in mouse models of demyelinating injuries. SIRT1 inactivation leads to the upregulation of genes involved in cell metabolism and growth factor signalling, in particular PDGF receptor α (PDGFRα). Oligodendrocyte expansion following SIRT1 inactivation is mediated at least in part by AKT and p38 MAPK-signalling molecules downstream of PDGFRα. The identification of drug-targetable enzymes that regulate oligodendrocyte regeneration in adults could facilitate the development of therapies for demyelinating injuries and diseases, such as multiple sclerosis.
The Journal of clinical investigation 2013 JUL
Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease.
Abstract
Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is caused by mutations in either PKD1 or PKD2 and is characterized by the development of multiple bilateral renal cysts that replace normal kidney tissue. Here, we used Pkd1 mutant mouse models to demonstrate that the nicotinamide adenine dinucleotide-dependent (NAD-dependent) protein deacetylase sirtuin 1 (SIRT1) is involved in the pathophysiology of ADPKD. SIRT1 was upregulated through c-MYC in embryonic and postnatal Pkd1-mutant mouse renal epithelial cells and tissues and could be induced by TNF-α, which is present in cyst fluid during cyst development. Double conditional knockouts of Pkd1 and Sirt1 demonstrated delayed renal cyst formation in postnatal mouse kidneys compared with mice with single conditional knockout of Pkd1. Furthermore, treatment with a pan-sirtuin inhibitor (nicotinamide) or a SIRT1-specific inhibitor (EX-527) delayed cyst growth in Pkd1 knockout mouse embryonic kidneys, Pkd1 conditional knockout postnatal kidneys, and Pkd1 hypomorphic kidneys. Increased SIRT1 expression in Pkd1 mutant renal epithelial cells regulated cystic epithelial cell proliferation through deacetylation and phosphorylation of Rb and regulated cystic epithelial cell death through deacetylation of p53. This newly identified role of SIRT1 signaling in cystic renal epithelial cells provides the opportunity to develop unique therapeutic strategies for ADPKD.
Proceedings of the National Academy of Sciences of the United States of America 2013 JUL
Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism.
Abstract
Abstract
Sirtuins are protein deacetylases regulating metabolism and stress responses. The seven human Sirtuins (Sirt1-7) are attractive drug targets, but Sirtuin inhibition mechanisms are mostly unidentified. We report the molecular mechanism of Sirtuin inhibition by 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide (Ex-527). Inhibitor binding to potently inhibited Sirt1 and Thermotoga maritima Sir2 and to moderately inhibited Sirt3 requires NAD(+), alone or together with acetylpeptide. Crystal structures of several Sirtuin inhibitor complexes show that Ex-527 occupies the nicotinamide site and a neighboring pocket and contacts the ribose of NAD(+) or of the coproduct 2'-O-acetyl-ADP ribose. Complex structures with native alkylimidate and thio-analog support its catalytic relevance and show, together with biochemical assays, that only the coproduct complex is relevant for inhibition by Ex-527, which stabilizes the closed enzyme conformation preventing product release. Ex-527 inhibition thus exploits Sirtuin catalysis, and kinetic isoform differences explain its selectivity. Our results provide insights in Sirtuin catalysis and inhibition with important implications for drug development.
Journal of biomolecular screening 2006 DEC
SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents.
Abstract
Abstract
The nicotinamide adenine dinucleotide (NAD(+))-dependent protein deacetylase SIRT1 has been linked to fatty acid metabolism via suppression of peroxysome proliferator-activated receptor gamma (PPAR-gamma) and to inflammatory processes by deacetylating the transcription factor NF-kappaB. First, modulation of SIRT1 activity affects lipid accumulation in adipocytes, which has an impact on the etiology of a variety of human metabolic diseases such as obesity and insulin-resistant diabetes. Second, activation of SIRT1 suppresses inflammation via regulation of cytokine expression. Using high-throughput screening, the authors identified compounds with SIRT1 activating and inhibiting potential. The biological activity of these SIRT1-modulating compounds was confirmed in cell-based assays using mouse adipocytes, as well as human THP-1 monocytes. SIRT1 activators were found to be potent lipolytic agents, reducing the overall lipid content of fully differentiated NIH L1 adipocytes. In addition, the same compounds have anti-inflammatory properties, as became evident by the reduction of the proinflammatory cytokine tumor necrosis factor-alpha (TNF-alpha). In contrast, a SIRT1 inhibitory compound showed a stimulatory activity on the differentiation of adipocytes, a feature often linked to insulin sensitization.