EC23
Retinoid pathway activator; Activates retinoic acid receptor (RAR)
概要
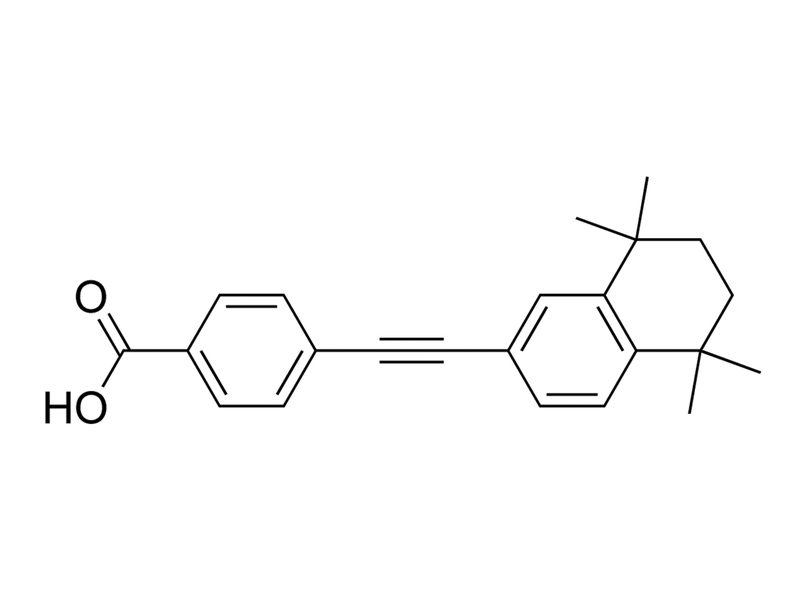
EC23 is a retinoic acid receptor (RAR) agonist with pan-RAR activity (EC₅₀: RARα 41 nM, RARβ 0.5 nM, RARγ 0.4 nM), while having no appreciable activity for retinoid X receptors (RXR; EC₅₀> 10μM for all; Gambone et al.). It is a photostable synthetic analog of all-trans retinoic acid (ATRA; Christie et al. 2008). EC23 also weakly activates aryl hydrocarbon receptor (Gambone et al.).
DIFFERENTIATION
· Induces neural differentiation of human pluripotent stem cells, similarly to ATRA (Christie et al. 2010; Clemens et al.).
· Induces neuronal differentiation of human fetal neural progenitor cell line ReNcell 197VM (Christie et al. 2010).
DIFFERENTIATION
· Induces neural differentiation of human pluripotent stem cells, similarly to ATRA (Christie et al. 2010; Clemens et al.).
· Induces neuronal differentiation of human fetal neural progenitor cell line ReNcell 197VM (Christie et al. 2010).
Alternative Names
AGN 190205; BASF 46928
Cell Type
Neural Cells, PSC-Derived, Neural Stem and Progenitor Cells, Neurons, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Neuroscience, Stem Cell Biology
CAS Number
104561-41-3
Chemical Formula
C₂₃H₂₄O₂
Molecular Weight
332.4 g/mol
Purity
≥ 98%
Pathway
Retinoid
Target
RAR
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EC23 | 73102, 73104 | All | English |
| Safety Data Sheet | EC23 | 73102, 73104 | All | English |
数据及文献
Publications (4)
Molecular bioSystems 2013
The action of all-trans-retinoic acid (ATRA) and synthetic retinoid analogues (EC19 and EC23) on human pluripotent stem cells differentiation investigated using single cell infrared microspectroscopy.
Abstract
Abstract
All trans-retinoic acid (ATRA) is widely used to direct the differentiation of cultured stem cells. When exposed to the pluripotent human embryonal carcinoma (EC) stem cell line, TERA2.cl.SP12, ATRA induces ectoderm differentiation and the formation of neuronal cell types. We have previously generated synthetic analogues of retinoic acid (EC23 and EC19) which also induce the differentiation of EC cells. Even though EC23 and EC19 have similar chemical structures, they have differing biochemical effects in terms of EC cell differentiation. EC23 induces neuronal differentiation in a manner similar to ATRA, whereas EC19 directs the cells to form epithelial-like derivatives. Previous MALDI-TOF MS analysis examined the response of TERA2.cl.SP12 cells after exposure to ATRA, EC23 and EC19 and further demonstrated the similarly in the effect of ATRA and EC23 activity whilst responses to EC19 were very different. In this study, we show that Fourier Transform Infrared Micro-Spectroscopy (FT-IRMS) coupled with appropriate scatter correction and multivariate analysis can be used as an effective tool to further investigate the differentiation of human pluripotent stem cells and monitor the alternative affects different retinoid compounds have on the induction of differentiation. FT-IRMS detected differences between cell populations as early as 3 days of compound treatment. Populations of cells treated with different retinoid compounds could easily be distinguished from one another during the early stages of cell differentiation. These data demonstrate that FT-IRMS technology can be used as a sensitive screening technique to monitor the status of the stem cell phenotype and progression of differentiation along alternative pathways in response to different compounds.
Journal of neuroscience methods 2010 NOV
Retinoid supplementation of differentiating human neural progenitors and embryonic stem cells leads to enhanced neurogenesis in vitro.
Abstract
Abstract
Retinoids are important molecules involved in the development and homeostasis of the nervous system. As such, various retinoid derivatives are often found in culture media and supplement formulations to support the growth and maintenance of neural cells. However, all-trans-retinoic acid (ATRA) and its associated derivatives are light sensitive and are highly susceptible to isomerisation. This can lead to variability in retinoid concentrations and the nature of the retinoid species present in culture solutions which in turn can influence biological activity and introduce inconsistency. We have previously described the development of the synthetic retinoid derivative, EC23, as a chemically and light stable alternative that does not degrade and has biological activity similar to ATRA. In this study we demonstrate that the addition of exogenous retinoid can significantly enhance neuronal differentiation of both human neuroprogenitor and human embryonic stem cells. In the former, both ATRA and EC23 induced increased maturation and stabilisation of the axonal cytoskeleton. However, EC23 was particularly potent at lower nanomolar concentrations resulting in significantly greater neurogenesis than ATRA. In ES cells enhanced motor neuron marker expression was also detected in response to both retinoids when incorporated into an established protocol for neuronal differentiation. We propose that synthetic retinoid EC23 represents a valuable addition to the formulation of new and existing culture supplements to enhance neuronal differentiation whilst enabling improved consistency.
Organic & biomolecular chemistry 2008 OCT
Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation.
Abstract
Abstract
All-trans-retinoic acid (ATRA) and its associated analogues are important mediators of cell differentiation and function during the development of the nervous system. It is well known that ATRA can induce the differentiation of neural tissues from human pluripotent stem cells. However, it is not always appreciated that ATRA is highly susceptible to isomerisation when in solution, which can influence the effective concentration of ATRA and subsequently its biological activity. To address this source of variability, synthetic retinoid analogues have been designed and synthesised that retain stability during use and maintain biological function in comparison to ATRA. It is also shown that subtle modifications to the structure of the synthetic retinoid compound impacts significantly on biological activity, as when exposed to cultured human pluripotent stem cells, synthetic retinoid 4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylethynyl)benzoic acid, 4a (para-isomer), induces neural differentiation similarly to ATRA. In contrast, stem cells exposed to synthetic retinoid 3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylethynyl)benzoic acid, 4b (meta-isomer), produce very few neurons and large numbers of epithelial-like cells. This type of structure-activity-relationship information for such synthetic retinoid compounds will further the ability to design more targeted systems capable of mediating robust and reproducible tissue differentiation.
Molecular pharmacology 2002
Unique property of some synthetic retinoids: activation of the aryl hydrocarbon receptor pathway.
Abstract
Abstract
Potential pharmacological applications in the areas of oncology, dermatology, diabetes, and atherosclerosis of synthetic analogs of retinoic acid that target a specific nuclear receptor and/or biological response have generated great interest in the development of new retinoid and rexinoid drugs. The pan-retinoic acid receptor antagonist AGN 193109 has been previously reported to elevate CYP1A1 levels, implicating the aryl hydrocarbon receptor (AhR) as an additional target for this retinoid. AhR is a cytosolic ligand-dependent transcription factor that, in conjunction with the AhR nuclear translocator (Arnt), binds to dioxin response elements (DREs) located in the promoter region of target genes, such as CYP1A1, and induces their transcription. The purpose of these studies was to determine whether additional synthetic retinoids were capable of elevating CYP1A1 levels and to examine the mechanism of this increase in CYP1A. Two additional retinoids, AGN 190730 and AGN 192837, were found to be potent inducers of DRE-driven transcriptional activity; AGN 190730 was the most potent. Moreover, electrophoretic mobility-shift assays demonstrate that AGN 190730 can transform AhR into its active DNA recognition form. In addition, trypsin digestion of AGN 190730-treated AhR reveals a conformational change in the protein similar to the conformational change of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-bound AhR. Finally, competitive binding studies demonstrate that AGN 190730 can inhibit the binding of TCDD to AhR. The sum of the data demonstrates that some synthetic retinoids in addition to activating the retinoic acid receptor/retinoid X receptor pathway are capable of binding to AhR and activating the AhR/Arnt pathway.