Doxorubicin
Anthracycline antibiotic; Inhibits DNA topoisomerase II
概要
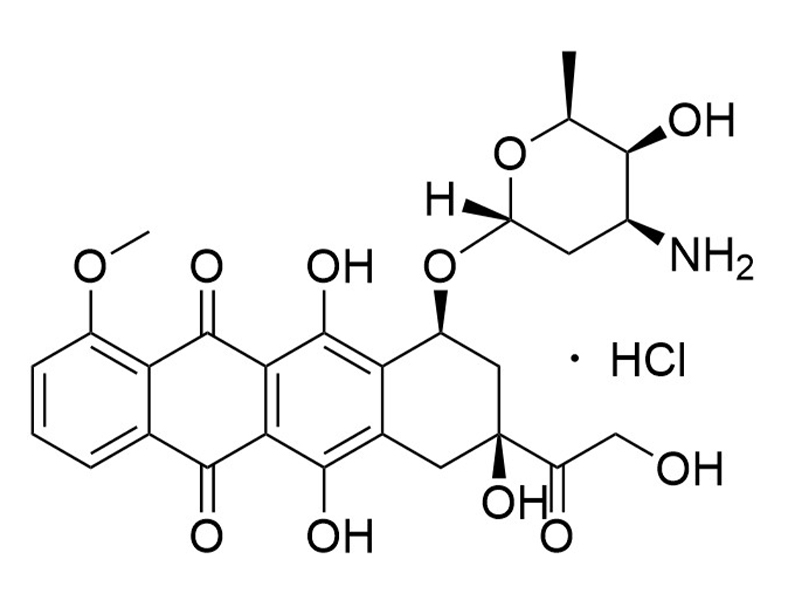
Doxorubicin is an anthracycline antibiotic that intercalates DNA and inhibits DNA topoisomerase II (Burridge et al.; Lorenzo et al.). Doxorubicin exhibits cardiotoxic effects that involve generating reactive oxygen species, inhibiting DNA topoisomerase II, and releasing calcium in the sarcoplasmic reticulum (Burridge et al.). This product is supplied as the hydrochloride salt of the molecule.
CANCER RESEARCH
· Induces apoptosis in human endothelial cells (Lorenzo et al.).
CANCER RESEARCH
· Induces apoptosis in human endothelial cells (Lorenzo et al.).
Alternative Names
DOX
Cell Type
Cancer Cells and Cell Lines, Endothelial Cells
Area of Interest
Cancer Research, Endothelial Cell Biology
CAS Number
25316-40-9
Chemical Formula
C27H29NO11 • HCl
Molecular Weight
580 g/mol
Purity
≥ 98%
Pathway
p53
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Doxorubicin (Hydrochloride) | 100-0558, 100-0559 | All | English |
| Safety Data Sheet | Doxorubicin (Hydrochloride) | 100-0558, 100-0559 | All | English |
数据及文献
Publications (2)
Nature medicine 2016
Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity.
Abstract
Abstract
Doxorubicin is an anthracycline chemotherapy agent effective in treating a wide range of malignancies, but it causes a dose-related cardiotoxicity that can lead to heart failure in a subset of patients. At present, it is not possible to predict which patients will be affected by doxorubicin-induced cardiotoxicity (DIC). Here we demonstrate that patient-specific human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) can recapitulate the predilection to DIC of individual patients at the cellular level. hiPSC-CMs derived from individuals with breast cancer who experienced DIC were consistently more sensitive to doxorubicin toxicity than hiPSC-CMs from patients who did not experience DIC, with decreased cell viability, impaired mitochondrial and metabolic function, impaired calcium handling, decreased antioxidant pathway activity, and increased reactive oxygen species production. Taken together, our data indicate that hiPSC-CMs are a suitable platform to identify and characterize the genetic basis and molecular mechanisms of DIC.
The Journal of biological chemistry 2002 mar
Doxorubicin induces apoptosis and CD95 gene expression in human primary endothelial cells through a p53-dependent mechanism.
Abstract
Abstract
Regulation of the homeostasis of vascular endothelium is critical for the processes of vascular remodeling and angiogenesis under physiological and pathological conditions. Here we show that doxorubicin (Dox), a drug used in antitumor therapy, triggered a marked accumulation of p53 and induced CD95 gene expression and apoptosis in proliferating human umbilical vein endothelial cells (HUVECs). Transfection and site-directed mutagenesis experiments using the CD95 promoter fused to an intronic enhancer indicated the requirement for a p53 site for Dox-induced promoter activation. Furthermore, the p53 inhibitor pifithrin-alpha (PFT-alpha) blocked both promoter inducibility and protein up-regulation of CD95 in response to Dox. Up-regulated CD95 in Dox-treated cells was functional in eliciting apoptosis upon incubation of the cells with an agonistic CD95 antibody. However, Dox-mediated apoptosis was independent of CD95/CD95L interaction. The analysis of apoptosis in the presence of PFT-alpha and benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone revealed that both p53 and caspase activation are required for Dox-mediated apoptosis of HUVECs. Finally, Dox triggered Bcl-2 down-regulation, cytochrome c release from mitochondria, and the activation of caspases 9 and 3, suggesting the involvement of a mitochondrially operated pathway of apoptosis. These results highlight the role of p53 in the response of primary endothelial cells to genotoxic drugs and may reveal a novel mechanism underlying the antitumoral properties of Dox, related to its ability to induce apoptosis in proliferating endothelial cells.