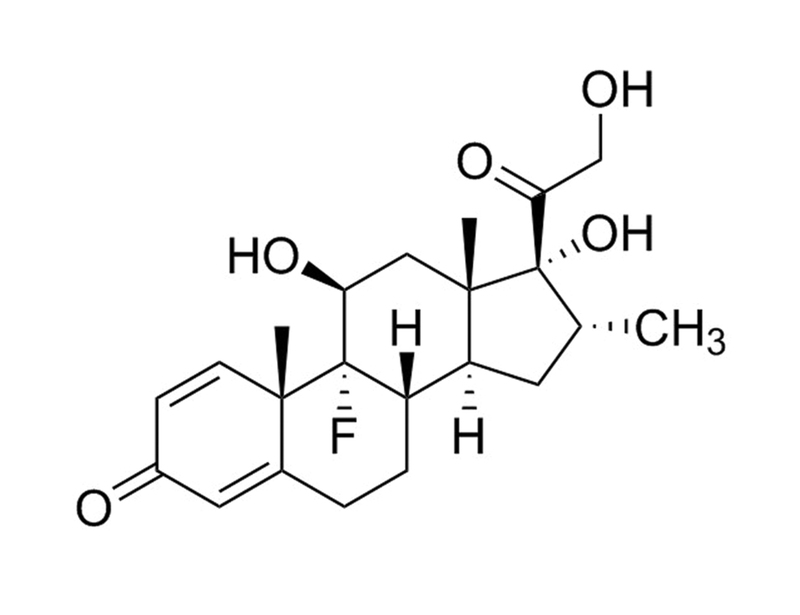
Dexamethasone
Glucocorticoid pathway activator; Activates glucocorticoid receptor
概要
Dexamethasone is a synthetic glucocorticoid, similar to the natural glucocorticoid hydrocortisone. Dexamethasone has an increased affinity for glucocorticoid receptors when compared to the natural hydrocortisone ligand (Kd = 5 nM vs 17 nM).
REPROGRAMMING
· Promotes transdifferentiation of hepatocytes from mouse pancreatic cells (Shen et al.).
DIFFERENTIATION
· Promotes osteogenic, adipogenic, and chondrogenic differentiation of human mesenchymal cells (Jaiswal et al., Mackay et al., Pittenger et al.).
· Promotes osteogenic, adipogenic, and chondrogenic differentiation of mouse mesenchymal cells (Tropel et al.).
· Promotes differentiation of mature hepatocytes from mouse and human embryonic stem (ES) cells (Cai et al., Kubo et al.).
· Promotes maturation of fetal mouse hepatocytes (Kamiya et al.).
REPROGRAMMING
· Promotes transdifferentiation of hepatocytes from mouse pancreatic cells (Shen et al.).
DIFFERENTIATION
· Promotes osteogenic, adipogenic, and chondrogenic differentiation of human mesenchymal cells (Jaiswal et al., Mackay et al., Pittenger et al.).
· Promotes osteogenic, adipogenic, and chondrogenic differentiation of mouse mesenchymal cells (Tropel et al.).
· Promotes differentiation of mature hepatocytes from mouse and human embryonic stem (ES) cells (Cai et al., Kubo et al.).
· Promotes maturation of fetal mouse hepatocytes (Kamiya et al.).
Alternative Names
MK 125; NSC 34521
Cell Type
Mesenchymal Stem and Progenitor Cells, Pancreatic Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Reprogramming
Area of Interest
Epithelial Cell Biology, Stem Cell Biology
CAS Number
50-02-2
Chemical Formula
C₂₂H₂₉FO₅
Molecular Weight
392.5 g/mol
Purity
≥ 98%
Pathway
Glucocorticoid
Target
Glucocorticoid Receptor
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | Dexamethasone | 72092 | All | English |
| Product Information Sheet 2 | Dexamethasone | 72092 | All | English |
| Safety Data Sheet | Dexamethasone | 72092 | All | English |
数据及文献
Publications (9)
Hepatology (Baltimore, Md.) 2007 MAY
Directed differentiation of human embryonic stem cells into functional hepatic cells.
Abstract
Abstract
UNLABELLED The differentiation capacity of human embryonic stem cells (hESCs) holds great promise for therapeutic applications. We report a novel three-stage method to efficiently direct the differentiation of human embryonic stem cells into hepatic cells in serum-free medium. Human ESCs were first differentiated into definitive endoderm cells by 3 days of Activin A treatment. Next, the presence of fibroblast growth factor-4 and bone morphogenetic protein-2 in the culture medium for 5 days induced efficient hepatic differentiation from definitive endoderm cells. Approximately 70% of the cells expressed the hepatic marker albumin. After 10 days of further in vitro maturation, these cells expressed the adult liver cell markers tyrosine aminotransferase, tryptophan oxygenase 2, phosphoenolpyruvate carboxykinase (PEPCK), Cyp7A1, Cyp3A4 and Cyp2B6. Furthermore, these cells exhibited functions associated with mature hepatocytes including albumin secretion, glycogen storage, indocyanine green, and low-density lipoprotein uptake, and inducible cytochrome P450 activity. When transplanted into CCl4 injured severe combined immunodeficiency mice, these cells integrated into the mouse liver and expressed human alpha-1 antitrypsin for at least 2 months. In addition, we found that the hESC-derived hepatic cells were readily infected by human immunodeficiency virus-hepatitis C virus pseudotype viruses. CONCLUSION We have developed an efficient way to direct the differentiation of human embryonic stem cells into cells that exhibit characteristics of mature hepatocytes. Our studies should facilitate searching the molecular mechanisms underlying human liver development, and form the basis for hepatocyte transplantation and drug tests.
Experimental cell research 2004 MAY
Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow.
Abstract
Abstract
The future use of adult mesenchymal stem cells (MSCs) for human therapies depends on the establishment of preclinical studies with other mammals such as mouse. Surprisingly, purification and characterisation of murine MSCs were only poorly documented. The aim of this study was to purify mouse MSCs from adult bone marrow and to functionally characterise their abilities to differentiate along diverse lineages. Adherent cells from adult C57Bl/6J mouse bone marrow were depleted of granulo-monocytic cells and subsequently allowed to grow on fibronectin-coated dishes in presence of fetal bovine serum and growth factors. The growing fibroblastoid cell population primarily consisted of spindle- and star-shaped cells with significant renewal capacity as they were cultured until 30 passages (about 60 doubling population). We fully demonstrated the MSC phenotype of these cells by inducing them to differentiate along osteoblastic, adipocytic, and chondrocytic pathways. Mouse MSCs (mMSCs) sharing the same morphological and functional characteristics as human MSCs can be successfully isolated from adult bone marrow without previous mouse or bone marrow treatment. Therefore, mMSCs will be an important tool to study the in vivo behaviour and fate of this cell type after grafting in mouse pathology models.
Development (Cambridge, England) 2004 APR
Development of definitive endoderm from embryonic stem cells in culture.
Abstract
Abstract
The cellular and molecular events regulating the induction and tissue-specific differentiation of endoderm are central to our understanding of the development and function of many organ systems. To define and characterize key components in this process, we have investigated the potential of embryonic stem (ES) cells to generate endoderm following their differentiation to embryoid bodies (EBs) in culture. We found that endoderm can be induced in EBs, either by limited exposure to serum or by culturing in the presence of activin A (activin) under serum-free conditions. By using an ES cell line with the green fluorescent protein (GFP) cDNA targeted to the brachyury locus, we demonstrate that endoderm develops from a brachyury(+) population that also displays mesoderm potential. Transplantation of cells generated from activin-induced brachyury(+) cells to the kidney capsule of recipient mice resulted in the development of endoderm-derived structures. These findings demonstrate that ES cells can generate endoderm in culture and, as such, establish this differentiation system as a unique murine model for studying the development and specification of this germ layer.
Nature cell biology 2000 DEC
Molecular basis of transdifferentiation of pancreas to liver.
Abstract
Abstract
The appearance of hepatic foci in the pancreas has been described in animal experiments and in human pathology. Here we show that pancreatic cells can be converted into hepatocytes by treatment with a synthetic glucocorticoid, dexamethasone. This occurs both in a pancreatic cell line, AR42J-B13, and in organ cultures of pancreatic buds from mouse embryos. We have established several features of the mechanism behind this transdifferentiation. We show that a proportion of the hepatocytes arises directly from differentiated exocrine-like cells, with no intervening cell division. This conversion is associated with induction of the transcription factor C/EBPbeta and the activation of differentiated hepatic products. Transfection of C/EBPbeta into the cells can provoke transdifferentiation; conversely, a dominant-negative form of C/EBPbeta can inhibit the process. These results indicate that C/EBPbeta is a key component that distinguishes the liver and pancreatic programmes of differentiation.
Science (New York, N.Y.) 1999 APR
Multilineage potential of adult human mesenchymal stem cells.
Abstract
Abstract
Human mesenchymal stem cells are thought to be multipotent cells, which are present in adult marrow, that can replicate as undifferentiated cells and that have the potential to differentiate to lineages of mesenchymal tissues, including bone, cartilage, fat, tendon, muscle, and marrow stroma. Cells that have the characteristics of human mesenchymal stem cells were isolated from marrow aspirates of volunteer donors. These cells displayed a stable phenotype and remained as a monolayer in vitro. These adult stem cells could be induced to differentiate exclusively into the adipocytic, chondrocytic, or osteocytic lineages. Individual stem cells were identified that, when expanded to colonies, retained their multilineage potential.
The EMBO journal 1999 APR
Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer.
Abstract
Abstract
Fetal liver, the major site of hematopoiesis during embryonic development, acquires additional various metabolic functions near birth. Although liver development has been characterized biologically as consisting of several distinct steps, the molecular events accompanying this process are just beginning to be characterized. In this study, we have established a novel culture system of fetal murine hepatocytes and investigated factors required for development of hepatocytes. We found that oncostatin M (OSM), an interleukin-6 family cytokine, in combination with glucocorticoid, induced maturation of hepatocytes as evidenced by morphological changes that closely resemble more differentiated hepatocytes, expression of hepatic differentiation markers and intracellular glycogen accumulation. Consistent with these in vitro observations, livers from mice deficient for gp130, an OSM receptor subunit, display defects in maturation of hepatocytes. Interestingly, OSM is expressed in CD45(+) hematopoietic cells in the developing liver, whereas the OSM receptor is expressed predominantly in hepatocytes. These results suggest a paracrine mechanism of hepatogenesis; blood cells, transiently expanding in the fetal liver, produce OSM to promote development of hepatocytes in vivo.