DAPT
Notch pathway inhibitor; Inhibits γ-secretase
概要
DAPT is an inhibitor of the γ-secretase complex. Notch is a key target of γ-secretase, therefore DAPT indirectly inhibits the Notch pathway. Other targets of γ-secretase that would be influenced by DAPT include amyloid precursor protein, E-cadherin, and ErbB4. (Dovey et al.).
MAINTENANCE AND SELF-RENEWAL
· Reduces colony-forming efficiency of mouse neural stem cells (Androutsellis-Theotokis et al.).
· Enhances radiation-induced cell death of glioma stem cells (Wang et al.).
DIFFERENTIATION
· Promotes differentiation of nociceptors from human pluripotent stem cells, in combination with several other small molecules (Chambers et al.).
· Promotes differentiation of neurons from human and mouse embryonic stem (ES) cells (Crawford and Roelink, Elkabetz et al.).
· Promotes differentiation of retinal pigment epithelium from mouse ES cells (Osakada et al.).
· Promotes differentiation of pancreatic cells from human pluripotent stem cells (D'Amour et al.).
CANCER RESEARCH
· Reduces mammosphere-forming efficiency of breast cancer cell lines and ductal carcinoma in situ cells (Farnie et al., Harrison et al.).
MAINTENANCE AND SELF-RENEWAL
· Reduces colony-forming efficiency of mouse neural stem cells (Androutsellis-Theotokis et al.).
· Enhances radiation-induced cell death of glioma stem cells (Wang et al.).
DIFFERENTIATION
· Promotes differentiation of nociceptors from human pluripotent stem cells, in combination with several other small molecules (Chambers et al.).
· Promotes differentiation of neurons from human and mouse embryonic stem (ES) cells (Crawford and Roelink, Elkabetz et al.).
· Promotes differentiation of retinal pigment epithelium from mouse ES cells (Osakada et al.).
· Promotes differentiation of pancreatic cells from human pluripotent stem cells (D'Amour et al.).
CANCER RESEARCH
· Reduces mammosphere-forming efficiency of breast cancer cell lines and ductal carcinoma in situ cells (Farnie et al., Harrison et al.).
Alternative Names
GSI-IX; LY-374973
Cell Type
Cancer Cells and Cell Lines, Endoderm, PSC-Derived, Mammary Cells, Neural Cells, PSC-Derived, Neural Stem and Progenitor Cells, Neurons, Pancreatic Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Cancer Research, Epithelial Cell Biology, Neuroscience, Stem Cell Biology
CAS Number
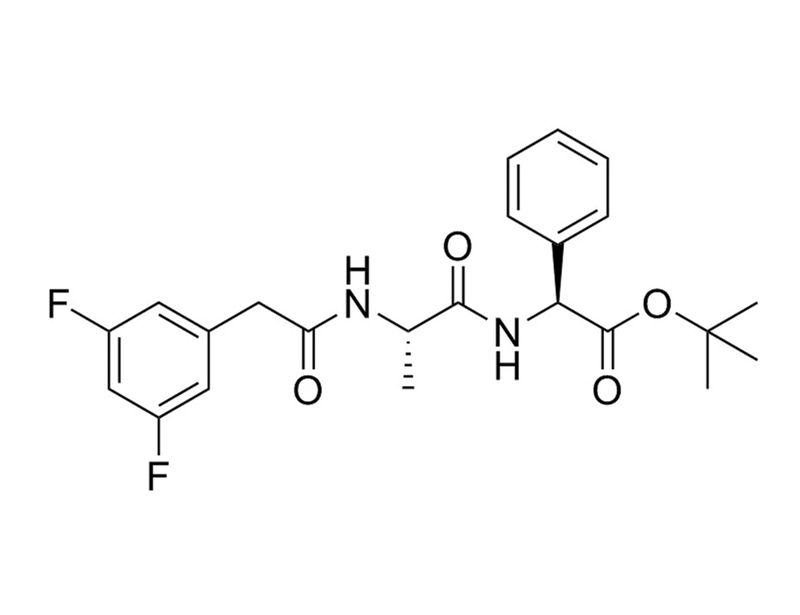
208255-80-5
Chemical Formula
C₂₃H₂₆F₂N₂O₄
Molecular Weight
432.5 g/mol
Purity
≥ 95%
Pathway
Notch
Target
γ-Secretase
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | DAPT | 72082 | All | English |
| Safety Data Sheet | DAPT | 72082 | All | English |
数据及文献
Publications (10)
Nature biotechnology 2012 JUL
Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors.
Abstract
Abstract
Considerable progress has been made in identifying signaling pathways that direct the differentiation of human pluripotent stem cells (hPSCs) into specialized cell types, including neurons. However, differentiation of hPSCs with extrinsic factors is a slow, step-wise process, mimicking the protracted timing of human development. Using a small-molecule screen, we identified a combination of five small-molecule pathway inhibitors that yield hPSC-derived neurons at textgreater75% efficiency within 10 d of differentiation. The resulting neurons express canonical markers and functional properties of human nociceptors, including tetrodotoxin (TTX)-resistant, SCN10A-dependent sodium currents and response to nociceptive stimuli such as ATP and capsaicin. Neuronal fate acquisition occurs about threefold faster than during in vivo development, suggesting that use of small-molecule pathway inhibitors could become a general strategy for accelerating developmental timing in vitro. The quick and high-efficiency derivation of nociceptors offers unprecedented access to this medically relevant cell type for studies of human pain.
Cancer research 2010 JAN
Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor.
Abstract
Abstract
Notch receptor signaling pathways play an important role not only in normal breast development but also in breast cancer development and progression. We assessed the role of Notch receptors in stem cell activity in breast cancer cell lines and nine primary human tumor samples. Stem cells were enriched by selection of anoikis-resistant cells or cells expressing the membrane phenotype ESA(+)/CD44(+)/CD24(low). Using these breast cancer stem cell populations, we compared the activation status of Notch receptors with the status in luminally differentiated cells, and we evaluated the consequences of pathway inhibition in vitro and in vivo. We found that Notch4 signaling activity was 8-fold higher in stem cell-enriched cell populations compared with differentiated cells, whereas Notch1 signaling activity was 4-fold lower in the stem cell-enriched cell populations. Pharmacologic or genetic inhibition of Notch1 or Notch4 reduced stem cell activity in vitro and reduced tumor formation in vivo, but Notch4 inhibition produced a more robust effect with a complete inhibition of tumor initiation observed. Our findings suggest that Notch4-targeted therapies will be more effective than targeting Notch1 in suppressing breast cancer recurrence, as it is initiated by breast cancer stem cells.
Stem cells (Dayton, Ohio) 2010 JAN
Notch promotes radioresistance of glioma stem cells.
Abstract
Abstract
Radiotherapy represents the most effective nonsurgical treatments for gliomas. However, gliomas are highly radioresistant and recurrence is nearly universal. Results from our laboratory and other groups suggest that cancer stem cells contribute to radioresistance in gliomas and breast cancers. The Notch pathway is critically implicated in stem cell fate determination and cancer. In this study, we show that inhibition of Notch pathway with gamma-secretase inhibitors (GSIs) renders the glioma stem cells more sensitive to radiation at clinically relevant doses. GSIs enhance radiation-induced cell death and impair clonogenic survival of glioma stem cells but not non-stem glioma cells. Expression of the constitutively active intracellular domains of Notch1 or Notch2 protect glioma stem cells against radiation. Notch inhibition with GSIs does not alter the DNA damage response of glioma stem cells after radiation but rather reduces Akt activity and Mcl-1 levels. Finally, knockdown of Notch1 or Notch2 sensitizes glioma stem cells to radiation and impairs xenograft tumor formation. Taken together, our results suggest a critical role of Notch signaling to regulate radioresistance of glioma stem cells. Inhibition of Notch signaling holds promise to improve the efficiency of current radiotherapy in glioma treatment.
Nature protocols 2009 JAN
Stepwise differentiation of pluripotent stem cells into retinal cells.
Abstract
Abstract
Embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass of blastocyst-stage embryos. They can maintain an undifferentiated state indefinitely and can differentiate into derivatives of all three germ layers, namely ectoderm, endoderm and mesoderm. Although much progress has been made in the propagation and differentiation of ES cells, induction of photoreceptors has generally required coculture with or transplantation into developing retinal tissue. Here, we describe a protocol for generating retinal cells from ES cells by stepwise treatment with defined factors. This method preferentially induces photoreceptor and retinal pigment epithelium (RPE) cells from mouse and human ES cells. In our protocol, differentiation of RPE and photoreceptors from mouse ES cells requires 28 d and the differentiation of human ES cells into mature RPE and photoreceptors requires 120 and 150 d, respectively. This differentiation system and the resulting pluripotent stem cell-derived retinal cells will facilitate the development of transplantation therapies for retinal diseases, drug testing and in vitro disease modeling. It will also improve our understanding of the development of the central nervous system, especially the eye.
Genes & development 2008 JAN
Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage.
Abstract
Abstract
Neural stem cells (NSCs) yield both neuronal and glial progeny, but their differentiation potential toward multiple region-specific neuron types remains remarkably poor. In contrast, embryonic stem cell (ESC) progeny readily yield region-specific neuronal fates in response to appropriate developmental signals. Here we demonstrate prospective and clonal isolation of neural rosette cells (termed R-NSCs), a novel NSC type with broad differentiation potential toward CNS and PNS fates and capable of in vivo engraftment. R-NSCs can be derived from human and mouse ESCs or from neural plate stage embryos. While R-NSCs express markers classically associated with NSC fate, we identified a set of genes that specifically mark the R-NSC state. Maintenance of R-NSCs is promoted by activation of SHH and Notch pathways. In the absence of these signals, R-NSCs rapidly lose rosette organization and progress to a more restricted NSC stage. We propose that R-NSCs represent the first characterized NSC stage capable of responding to patterning cues that direct differentiation toward region-specific neuronal fates. In addition, the R-NSC-specific genetic markers presented here offer new tools for harnessing the differentiation potential of human ESCs.
Developmental dynamics : an official publication of the American Association of Anatomists 2007 MAR
The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling.
Abstract
Abstract
During development of the neural tube, inhibition of the Notch response as well as the activation of the Sonic Hedgehog (Shh) response results in the formation of neuronal cell types. To determine whether Shh and Notch act independently, we tested the effects of the Notch inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) on neuralized, embryonic stem (ES) cell-derived embryoid bodies (EBs), while varying the levels of Shh pathway activation. Shh-resistant EBs were derived from Smo null ES cells, while EBs with constitutive high level of Shh pathway activation were derived from Ptc1 null ES cells. Intermediate levels of Shh pathway activation was achieved by the addition of ShhN to the EB culture medium. It was found that DAPT-mediated inhibition of the Notch response resulted in enhanced neuronal differentiation. In the absence of Shh, more interneurons were detected, while the main effect of DAPT on EBs with an activated Shh response was the precocious loss of ventral neuronal precursor-specific markers.