Cardiogenol C
Inducer of cardiac differentiation
概要
Cardiogenol C is a diaminopyrimidine that induces cardiomyogenesis in mouse embryonic stem cells. Its use results in expression of GATA-4, MEF2, and Nkx2.5.
REPROGRAMMING
· Induces trans-differentiation of mouse CD34+K15+ hair bulge progenitor cells into cardiomyocyte-like cells (Yau et al.).
· Induces cardiomyogenic function in the lineage-committed progenitor cells, C2C12 skeletal myoblasts and mouse A5 cardiovascular progenitor cells (Mike et al.).
DIFFERENTIATION
· Induces the differentiation of myosin heavy chain (MHC)-positive cardiomyocytes from mouse embryonic stem cells (Wu et al.).
REPROGRAMMING
· Induces trans-differentiation of mouse CD34+K15+ hair bulge progenitor cells into cardiomyocyte-like cells (Yau et al.).
· Induces cardiomyogenic function in the lineage-committed progenitor cells, C2C12 skeletal myoblasts and mouse A5 cardiovascular progenitor cells (Mike et al.).
DIFFERENTIATION
· Induces the differentiation of myosin heavy chain (MHC)-positive cardiomyocytes from mouse embryonic stem cells (Wu et al.).
Alternative Names
Cardiogenol C; Cardiogenol C hydrochloride
Cell Type
Cardiomyocytes, PSC-Derived, Myogenic Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
671225-39-1
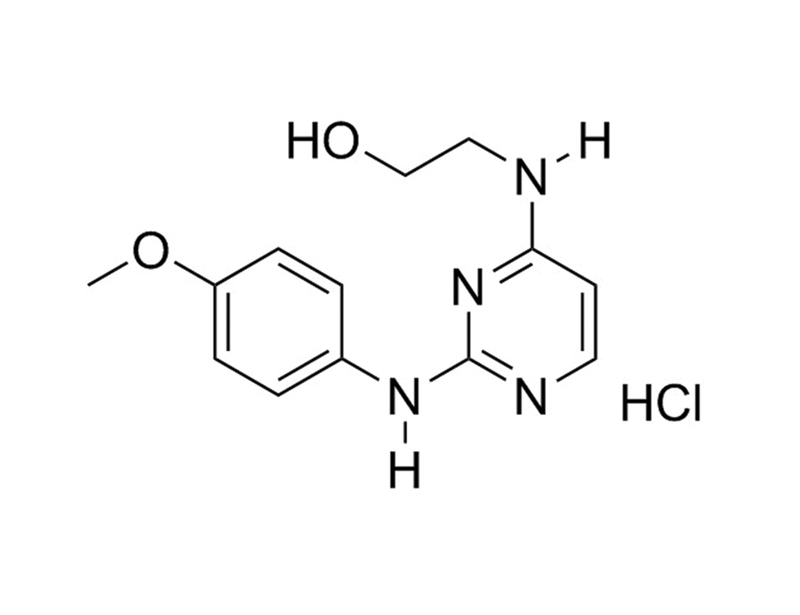
Chemical Formula
C₁₃H₁₆N₄O₂ · HCl
Molecular Weight
296.8 g/mol
Purity
≥ 97%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Cardiogenol C (Hydrochloride) | 72422, 72424 | All | English |
| Safety Data Sheet | Cardiogenol C (Hydrochloride) | 72422, 72424 | All | English |
数据及文献
Publications (3)
Cellular physiology and biochemistry 2014 JAN
Small molecule cardiogenol C upregulates cardiac markers and induces cardiac functional properties in lineage-committed progenitor cells.
Abstract
Abstract
BACKGROUND/AIMS: Cell transplantation into the heart is a new therapy after myocardial infarction. Its success, however, is impeded by poor donor cell survival and by limited transdifferentiation of the transplanted cells into functional cardiomyocytes. A promising strategy to overcome these problems is the induction of cardiomyogenic properties in donor cells by small molecules. METHODS: Here we studied cardiomyogenic effects of the small molecule compound cardiogenol C (CgC), and structural derivatives thereof, on lineage-committed progenitor cells by various molecular biological, biochemical, and functional assays. RESULTS: Treatment with CgC up-regulated cardiac marker expression in skeletal myoblasts. Importantly, the compound also induced cardiac functional properties: first, cardiac-like sodium currents in skeletal myoblasts, and secondly, spontaneous contractions in cardiovascular progenitor cell-derived cardiac bodies. CONCLUSION: CgC induces cardiomyogenic function in lineage-committed progenitor cells, and can thus be considered a promising tool to improve cardiac repair by cell therapy.
Proteome science 2011 JAN
Cardiogenol C can induce Mouse Hair Bulge Progenitor Cells to Transdifferentiate into Cardiomyocyte-like Cells.
Abstract
Abstract
BACKGROUND: Hair bulge progenitor cells (HBPCs) are multipotent stem cells derived from the bulge region of mice vibrissal hairs. The purified HBPCs express CD34, K15 and K14 surface markers. It has been reported that HBPCs could be readily induced to transdifferentiate into adipocytes and osteocytes. However, the ability of HBPCs to transdifferentiate into cardiomyocytes has not yet been investigated. METHODOLOGY/PRINCIPAL FINDINGS: The cardiomyogenic potential of HBPCs was investigated using a small cell-permeable molecule called Cardiogenol C. We established that Cardiogenol C could induce HBPCs to express transcription factors GATA4, Nkx2.5 and Tbx5, which are early specific markers for pre-cardiomyogenic cells. In prolonged cultures, the Cardiogenol C-treated HBPCs can also express muscle proteins, cardiac-specific troponin I and sarcomeric myosin heavy chain. However, we did not observe the ability of these cells to functionally contract. Hence, we called these cells cardiomyocyte-like cells rather than cardiomyocytes. We tried to remedy this deficiency by pre-treating HBPCs with Valproic acid first before exposing them to Cardiogenol C. This pretreatment inhibited, rather than improved, the effectiveness of Cardiogenol C in reprogramming the HBPCs. We used comparative proteomics to determine how Cardiogenol C worked by identifying proteins that were differentially expressed. We identified proteins that were involved in promoting cell differentiation, cardiomyocyte development and for the normal function of striated muscles. From those differentially expressed proteins, we further propose that Cardiogenol C might exert its effect by activating the Wnt signaling pathway through the suppression of Kremen1. In addition, by up-regulating the expression of chromatin remodeling proteins, SIK1 and Smarce1 would initiate cardiac differentiation. CONCLUSIONS/SIGNIFICANCE: In conclusion, our CD34+/K15+ HBPCs could be induced to transdifferentiate into cardiomyocyte-like cells using a small molecule called Cardiogenol C. The process involves activation of the Wnt signaling pathway and altered expression of several key chromatin remodeling proteins. The finding is clinically significant as HBPCs offer a readily accessible and autologous source of progenitor cells for cell-based therapy of heart disease, which is one of major killers in developed countries.
Journal of the American Chemical Society 2004 FEB
Small molecules that induce cardiomyogenesis in embryonic stem cells.
Abstract
Abstract
A phenotypic cell-based screen of a large combinatorial chemical library led to the identification of a class of diaminopyrimidine compounds (cardiogenol A-D) which can selectively and efficiently induce mouse embryonic stem cells (ESCs) to differentiate into cardiomyocytes. ESC-derived cardiomyocytes were shown to express multiple cardiac muscle markers, including myosin heavy chain, GATA-4, MEF2, and Nkx2.5, and spontaneously form beating regions. Such small molecules will serve as useful chemical probes to study cardiac muscle differentiation and may ultimately facilitate the therapeutic application of ESCs for cardiac repair.