CHIR98014
WNT pathway activator; Inhibits GSK3α and GSK3ß
概要
CHIR98014 is a reversible, cell-permeable activator of the WNT pathway, through inhibition of both isoforms of glycogen synthase kinase 3 (GSK3α and GSK3β) with IC₅₀ values of 0.65 and 0.58 nM, respectively. It shows at least 500-fold selectivity for GSK-3 versus 20 other serine/threonine or tyrosine kinases (Ring et al.).
DIFFERENTIATION
· Induces differentiation of endothelial progenitor cells from human induced pluripotent stem cells in the absence of VEGF (Lian et al.).
· Enhances osteogenic-like differentiation of rat mesenchymal stem cells cultured with high phosphate, including BMP-2 expression, calcium deposition, and alkaline phosphatase activity (Guerrero et al.).
METABOLISM
· Activates glycogen synthase in cells, lowers blood glucose levels and improves glucose disposal in insulin-resistant rats and diabetic db/db mice (Ring et al.).
DISEASE MODELING
· Reduces tau phosphorylation in the cortex and hippocampus of postnatal rat brains, which is implicated in the formation of neurofibrillary tangles and defects in axonal transport in Alzheimer’s disease (Selenica et al.).
DIFFERENTIATION
· Induces differentiation of endothelial progenitor cells from human induced pluripotent stem cells in the absence of VEGF (Lian et al.).
· Enhances osteogenic-like differentiation of rat mesenchymal stem cells cultured with high phosphate, including BMP-2 expression, calcium deposition, and alkaline phosphatase activity (Guerrero et al.).
METABOLISM
· Activates glycogen synthase in cells, lowers blood glucose levels and improves glucose disposal in insulin-resistant rats and diabetic db/db mice (Ring et al.).
DISEASE MODELING
· Reduces tau phosphorylation in the cortex and hippocampus of postnatal rat brains, which is implicated in the formation of neurofibrillary tangles and defects in axonal transport in Alzheimer’s disease (Selenica et al.).
Alternative Names
Not applicable
Cell Type
Endothelial Cells, Mesenchymal Stem and Progenitor Cells, Mesoderm, PSC-Derived, Osteoblasts, Pancreatic Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Disease Modeling, Neuroscience, Stem Cell Biology
CAS Number
556813-39-9
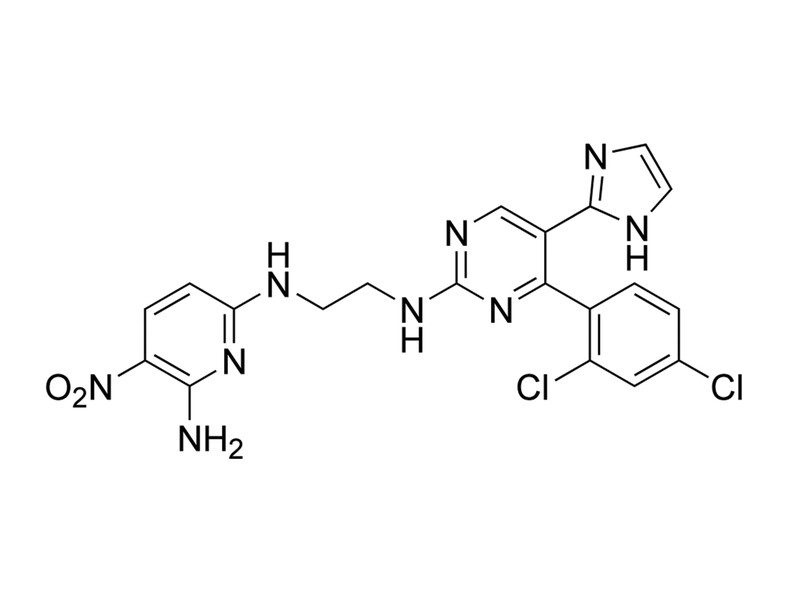
Chemical Formula
C₂₀H₁₇Cl₂N₉O₂
Molecular Weight
486.3 g/mol
Purity
≥ 98%
Pathway
WNT
Target
GSK3
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CHIR98014 | 73042, 73044 | All | English |
| Safety Data Sheet | CHIR98014 | 73042, 73044 | All | English |
数据及文献
Publications (4)
Stem cell reports 2014 NOV
Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling.
Abstract
Abstract
Human pluripotent stem cell (hPSC)-derived endothelial cells and their progenitors may provide the means for vascularization of tissue-engineered constructs and can serve as models to study vascular development and disease. Here, we report a method to efficiently produce endothelial cells from hPSCs via GSK3 inhibition and culture in defined media to direct hPSC differentiation to CD34(+)CD31(+) endothelial progenitors. Exogenous vascular endothelial growth factor (VEGF) treatment was dispensable, and endothelial progenitor differentiation was β-catenin dependent. Furthermore, by clonal analysis, we showed that CD34(+)CD31(+)CD117(+)TIE-2(+) endothelial progenitors were multipotent, capable of differentiating into calponin-expressing smooth muscle cells and CD31(+)CD144(+)vWF(+)I-CAM1(+) endothelial cells. These endothelial cells were capable of 20 population doublings, formed tube-like structures, imported acetylated low-density lipoprotein, and maintained a dynamic barrier function. This study provides a rapid and efficient method for production of hPSC-derived endothelial progenitors and endothelial cells and identifies WNT/β-catenin signaling as a primary regulator for generating vascular cells from hPSCs.
PloS one 2014
TGF-β prevents phosphate-induced osteogenesis through inhibition of BMP and Wnt/β-catenin pathways.
Abstract
Abstract
BACKGROUND: Transforming growth factor-β (TGF-β) is a key cytokine during differentiation of mesenchymal stem cells (MSC) into vascular smooth muscle cells (VSMC). High phosphate induces a phenotypic transformation of vascular smooth muscle cells (VSMC) into osteogenic-like cells. This study was aimed to evaluate signaling pathways involved during VSMC differentiation of MSC in presence or not of high phosphate. RESULTS: Our results showed that TGF-β induced nuclear translocation of Smad3 as well as the expression of vascular smooth muscle markers, such as smooth muscle alpha actin, SM22α, myocardin, and smooth muscle-myosin heavy chain. The addition of high phosphate to MSC promoted nuclear translocation of Smad1/5/8 and the activation of canonical Wnt/β-catenin in addition to an increase in BMP-2 expression, calcium deposition and alkaline phosphatase activity. The administration of TGF-β to MSC treated with high phosphate abolished all these effects by inhibiting canonical Wnt, BMP and TGF-β pathways. A similar outcome was observed in high phosphate-treated cells after the inhibition of canonical Wnt signaling with Dkk-1. Conversely, addition of both Wnt/β-catenin activators CHIR98014 and lithium chloride enhanced the effect of high phosphate on BMP-2, calcium deposition and alkaline phosphatase activity. CONCLUSIONS: Full VSMC differentiation induced by TGF-β may not be achieved when extracellular phosphate levels are high. Moreover, TGF-β prevents high phosphate-induced osteogenesis by decreasing the nuclear translocation of Smad 1/5/8 and avoiding the activation of Wnt/β-catenin pathway.
British journal of pharmacology 2007
Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation.
Abstract
Abstract
BACKGROUND AND PURPOSE: Glycogen synthase kinase-3 (GSK-3) affects neuropathological events associated with Alzheimeŕs disease (AD) such as hyperphosphorylation of the protein, tau. GSK-3beta expression, enzyme activity and tau phosphorylated at AD-relevant epitopes are elevated in juvenile rodent brains. Here, we assess five GSK-3beta inhibitors and lithium in lowering phosphorylated tau (p-tau) and GSK-3beta enzyme activity levels in 12-day old postnatal rats. EXPERIMENTAL APPROACH: Brain levels of inhibitors following treatment in vivo were optimized based on pharmacokinetic data. At optimal doses, p-tau (Ser(396)) levels in brain tissue was measured by immunoblotting and correlated with GSK-3beta enzyme activities in the same tissues. Effects of GSK inhibitors on p-tau, GSK-3beta activities and cell death were measured in a human neuronal cell line (LUHMES). KEY RESULTS: Lithium and CHIR98014 reduced tau phosphorylation (Ser(396)) in the cortex and hippocampus of postnatal rats, while Alsterpaullone and SB216763 were effective only in hippocampus. AR-A014418 and Indirubin-3'-monoxime were ineffective in either brain region. Inhibition of p-tau in brain required several-fold higher levels of GSK inhibitors than the IC(50) values obtained in recombinant or cell-based GSK-3beta enzyme activity assays. The inhibitory effect on GSK-3beta activity ex vivo correlated with protection against cell death and decrease of p-tau- in LUHMES cells, using low microM inhibitor concentrations. CONCLUSIONS AND IMPLICATIONS: Selective small-molecule inhibitors of GSK-3 reduce tau phosphorylation in vivo. These findings corroborate earlier suggestions that GSK-3beta may be an attractive target for disease-modification in AD and related conditions where tau phosphorylation is believed to contribute to disease pathogenesis.
Diabetes 2003
Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo.
Abstract
Abstract
Insulin resistance plays a central role in the development of type 2 diabetes, but the precise defects in insulin action remain to be elucidated. Glycogen synthase kinase 3 (GSK-3) can negatively regulate several aspects of insulin signaling, and elevated levels of GSK-3 have been reported in skeletal muscle from diabetic rodents and humans. A limited amount of information is available regarding the utility of highly selective inhibitors of GSK-3 for the modification of insulin action under conditions of insulin resistance. In the present investigation, we describe novel substituted aminopyrimidine derivatives that inhibit human GSK-3 potently (K(i) textless 10 nmol/l) with at least 500-fold selectivity against 20 other protein kinases. These low molecular weight compounds activated glycogen synthase at approximately 100 nmol/l in cultured CHO cells transfected with the insulin receptor and in primary hepatocytes isolated from Sprague-Dawley rats, and at 500 nmol/l in isolated type 1 skeletal muscle of both lean Zucker and ZDF rats. It is interesting that these GSK-3 inhibitors enhanced insulin-stimulated glucose transport in type 1 skeletal muscle from the insulin-resistant ZDF rats but not from insulin-sensitive lean Zucker rats. Single oral or subcutaneous doses of the inhibitors (30-48 mg/kg) rapidly lowered blood glucose levels and improved glucose disposal after oral or intravenous glucose challenges in ZDF rats and db/db mice, without causing hypoglycemia or markedly elevating insulin. Collectively, our results suggest that these selective GSK-3 inhibitors may be useful as acute-acting therapeutics for the treatment of the insulin resistance of type 2 diabetes.