CFTR(inh)-172
Inhibits cystic fibrosis transmembrane conductance regulator (CFTR)
概要
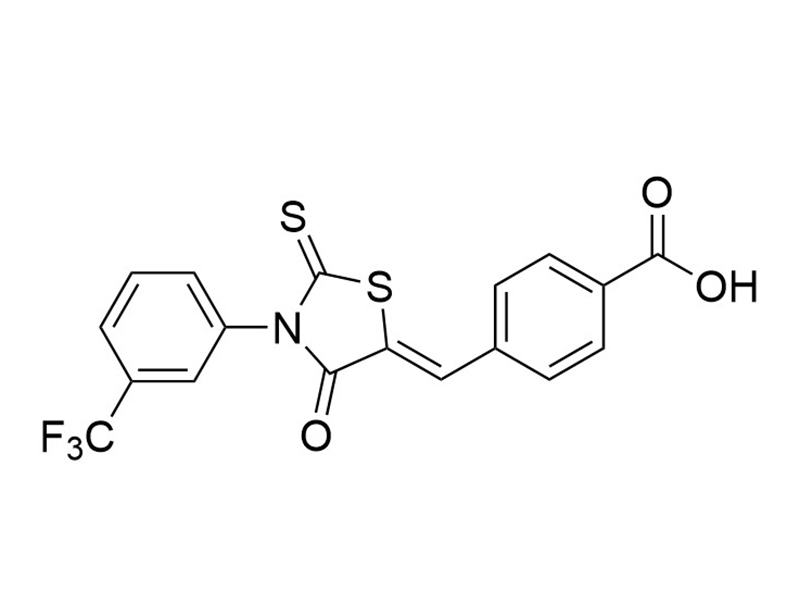
CFTR(inh)-172 is a selective and reversible thiazolidinone inhibitor of the cystic fibrosis transmembrane conductance regulator (CFTR; Ki = 300 nM; Ma et al.). CFTR is a chloride anion channel involved in the secretion of fluid in many epithelial tissues, such as the airway and intestine (Ma et al.). Defects in the CFTR gene alter ion transport, which can lead to cystic fibrosis (Dalli et al.; Ma et al.). When it is intraperitoneally injected in mice, CFTR(inh)-172 inhibits the secretion of intestinal fluid induced by cholera toxin (Ma et al.).
DISEASE MODELING
· Slows cyst growth in polycystic kidney disease in mice (Yang et al.).
DISEASE MODELING
· Slows cyst growth in polycystic kidney disease in mice (Yang et al.).
Alternative Names
Cystic fibrosis transmembrane conductance regulator inhibitor 172; CFTR Inhibitor-172
Cell Type
Airway Cells, Intestinal Cells
Area of Interest
Disease Modeling, Epithelial Cell Biology
CAS Number
307510-92-5
Chemical Formula
C18H10F3NO3S2
Molecular Weight
409.4 g/mol
Purity
≥ 98%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CFTR(inh)-172 | 100-0554, 100-0555 | All | English |
| Safety Data Sheet | CFTR(inh)-172 | 100-0554, 100-0555 | All | English |
数据及文献
Publications (3)
The American journal of pathology 2010 jul
CFTR inhibition provokes an inflammatory response associated with an imbalance of the annexin A1 pathway.
Abstract
Abstract
Cystic fibrosis (CF), a disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, is characterized by chronic bacterial infections and inflammation in the lung. Having previously shown that deletion of CFTR is associated with lower expression of the endogenous anti-inflammatory protein Annexin A1 (AnxA1), we investigated further this possible functional connection using a validated CFTR inhibitor. Treatment of mice with the CFTR inhibitor-172 (CFTR(172)) augmented the acute peritonitis promoted by zymosan, an effect associated with lower AnxA1 levels in peritoneal cells. Similar results were obtained with another, chemically distinct, CFTR inhibitor. The pro-inflammatory effect of CFTR(172) was lost in AnxA1(-/-), as well as CFTR(-/-) mice. Importantly, administration of hrAnxA1 and its peptido-mimetic to CFTR(-/-) animals or to animals treated with CFTR(172) corrected the exaggerated leukocyte migration seen in these animals. In vitro assays with human Polymorphonuclear leukocyte (PMN) demonstrated that CFTR(172) reduced cell-associated AnxA1 by promoting release of the protein in microparticles. We propose that the reduced impact of the counterregulatory properties of AnxA1 in CF cells contributes to the inflammatory phenotype characteristic of this disease. Thus, these findings provide an important insight into the mechanism underlying the inflammatory disease associated with CFTR inhibition while, at the same time, providing a novel pharmacological target for controlling the inflammatory phenotype of CF.
Journal of the American Society of Nephrology : JASN 2008 jul
Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease.
Abstract
Abstract
Cyst expansion in polycystic kidney disease (PKD) involves progressive fluid accumulation, which is believed to require chloride transport by the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Herein is reported that small-molecule CFTR inhibitors of the thiazolidinone and glycine hydrazide classes slow cyst expansion in in vitro and in vivo models of PKD. More than 30 CFTR inhibitor analogs were screened in an MDCK cell model, and near-complete suppression of cyst growth was found by tetrazolo-CFTR(inh)-172, a tetrazolo-derived thiazolidinone, and Ph-GlyH-101, a phenyl-derived glycine hydrazide, without an effect on cell proliferation. These compounds also inhibited cyst number and growth by {\textgreater}80{\%} in an embryonic kidney cyst model involving 4-d organ culture of embryonic day 13.5 mouse kidneys in 8-Br-cAMP-containing medium. Subcutaneous delivery of tetrazolo-CFTR(inh)-172 and Ph-GlyH-101 to neonatal, kidney-specific PKD1 knockout mice produced stable, therapeutic inhibitor concentrations of {\textgreater}3 microM in urine and kidney tissue. Treatment of mice for up to 7 d remarkably slowed kidney enlargement and cyst expansion and preserved renal function. These results implicate CFTR in renal cyst growth and suggest that CFTR inhibitors may hold therapeutic potential to reduce cyst growth in PKD.
The Journal of clinical investigation 2002 dec
Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion.
Abstract
Abstract
Secretory diarrhea is the leading cause of infant death in developing countries and a major cause of morbidity in adults. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is required for fluid secretion in the intestine and airways and, when defective, causes the lethal genetic disease cystic fibrosis. We screened 50,000 chemically diverse compounds for inhibition of cAMP/flavone-stimulated Cl(-) transport in epithelial cells expressing CFTR. Six CFTR inhibitors of the 2-thioxo-4-thiazolidinone chemical class were identified. The most potent compound discovered by screening of structural analogs, CFTR(inh)-172, reversibly inhibited CFTR short-circuit current in less than 2 minutes in a voltage-independent manner with K(I) approximately 300 nM. CFTR(inh)-172 was nontoxic at high concentrations in cell culture and mouse models. At concentrations fully inhibiting CFTR, CFTR(inh)-172 did not prevent elevation of cellular cAMP or inhibit non-CFTR Cl(-) channels, multidrug resistance protein-1 (MDR-1), ATP-sensitive K(+) channels, or a series of other transporters. A single intraperitoneal injection of CFTR(inh)-172 (250 micro g/kg) in mice reduced by more than 90{\%} cholera toxin-induced fluid secretion in the small intestine over 6 hours. Thiazolidinone CFTR inhibitors may be useful in developing large-animal models of cystic fibrosis and in reducing intestinal fluid loss in cholera and other secretory diarrheas.