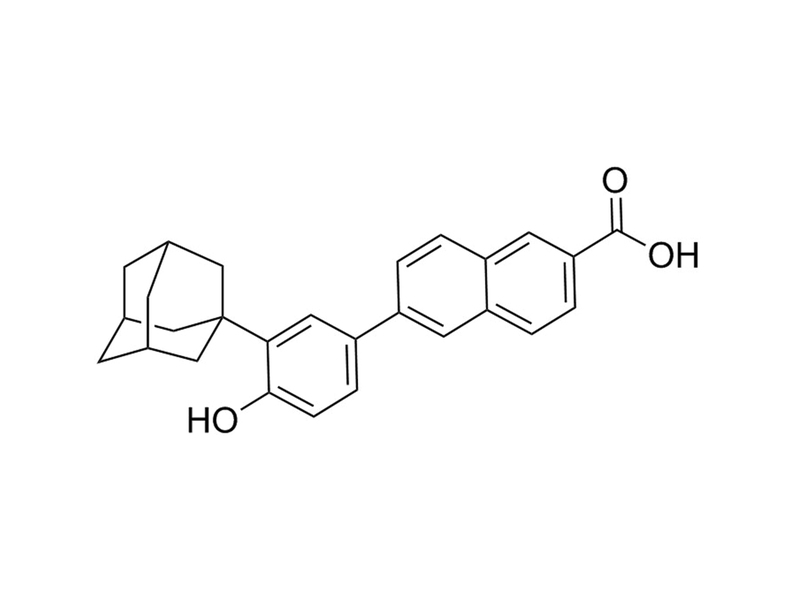
CD437
Retinoid pathway activator; Activates retinoic acid receptor (RAR)
概要
CD437 is the prototypical adamantyl arotinoid of the retinoid-related molecule family that acts as a selective agonist of retinoic acid receptor (RAR)γ (Kd = 6.5 µM, 2.5 µM, and 77 nM for RARα, β, and γ, respectively; Bernard et al.; Pérez-Rodríguez et al.).
REPROGRAMMING
· Increases speed and number of pre-induced pluripotent stem (iPS) cell colonies generated from mouse embryonic fibroblasts (MEFs) transfected with Oct4, Sox2, c-Myc, and Klf4 (Wang et al.).
CANCER RESEARCH
· Induces cell cycle arrest and apoptosis in a variety of cancer cells (Fontana and Rishi; Jin et al.; Li et al.; Valli et al.)
· Decreases mRNA expression of squamous differentiation markers cytokeratin 1, involucrin, and SPR1 in the human head and neck squamous cell carcinoma cell line, UMSCC22B (Sun et al.).
REPROGRAMMING
· Increases speed and number of pre-induced pluripotent stem (iPS) cell colonies generated from mouse embryonic fibroblasts (MEFs) transfected with Oct4, Sox2, c-Myc, and Klf4 (Wang et al.).
CANCER RESEARCH
· Induces cell cycle arrest and apoptosis in a variety of cancer cells (Fontana and Rishi; Jin et al.; Li et al.; Valli et al.)
· Decreases mRNA expression of squamous differentiation markers cytokeratin 1, involucrin, and SPR1 in the human head and neck squamous cell carcinoma cell line, UMSCC22B (Sun et al.).
Alternative Names
AHPN
Cell Type
Cancer Cells and Cell Lines, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Reprogramming
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
125316-60-1
Chemical Formula
C₂₇H₂₆O₃
Molecular Weight
398.5 g/mol
Purity
≥ 95%
Pathway
Retinoid
Target
RAR
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CD437 | 72722, 72724 | All | English |
| Safety Data Sheet | CD437 | 72722, 72724 | All | English |
数据及文献
Publications (8)
Proceedings of the National Academy of Sciences of the United States of America 2011 NOV
Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1.
Abstract
Abstract
Somatic cells can be reprogrammed to induced pluripotent stem cells (iPSCs) by expressing four transcription factors: Oct4, Sox2, Klf4, and c-Myc. Here we report that enhancing RA signaling by expressing RA receptors (RARs) or by RA agonists profoundly promoted reprogramming, but inhibiting it using a RAR-α dominant-negative form completely blocked it. Coexpressing Rarg (RAR-γ) and Lrh-1 (liver receptor homologue 1; Nr5a2) with the four factors greatly accelerated reprogramming so that reprogramming of mouse embryonic fibroblast cells to ground-state iPSCs requires only 4 d induction of these six factors. The six-factor combination readily reprogrammed primary human neonatal and adult fibroblast cells to exogenous factor-independent iPSCs, which resembled ground-state mouse ES cells in growth properties, gene expression, and signaling dependency. Our findings demonstrate that signaling through RARs has critical roles in molecular reprogramming and that the synergistic interaction between Rarg and Lrh1 directs reprogramming toward ground-state pluripotency. The human iPSCs described here should facilitate functional analysis of the human genome.
European journal of medicinal chemistry 2009 JUN
Highly twisted adamantyl arotinoids: synthesis, antiproliferative effects and RXR transactivation profiles.
Abstract
Abstract
Retinoid-related molecules with an adamantyl group (adamantyl arotinoids) have been described with selective activities towards the retinoid receptors as agonists for NR1B2 and NR1B3 (RARbeta,gamma) (CD437, MX3350-1) or RAR antagonists (MX781) that induce growth arrest and apoptosis in cancer cells. Since these molecules induce apoptosis independently of RAR transactivation, we set up to synthesize novel analogs with impaired RAR binding. Here we describe adamantyl arotinoids with 2,2'-disubstituted biaryl rings prepared using the Suzuki coupling of the corresponding fragments. Those with cinnamic and naphthoic acid end groups showed significant antiproliferative activity in several cancer cell lines, and this effect correlated with the induction of apoptosis as measured by caspase activity. Strikingly, some of these compounds, whereas devoid of RAR binding capacity, were able to activate RXR.
Molecular cancer therapeutics 2008 SEP
Atypical retinoids ST1926 and CD437 are S-phase-specific agents causing DNA double-strand breaks: significance for the cytotoxic and antiproliferative activity.
Abstract
Abstract
Retinoid-related molecules (RRM) are novel agents with tumor-selective cytotoxic/antiproliferative activity, a different mechanism of action from classic retinoids and no cross-resistance with other chemotherapeutics. ST1926 and CD437 are prototypic RRMs, with the former currently undergoing phase I clinical trials. We show here that ST1926, CD437, and active congeners cause DNA damage. Cellular and subcellular COMET assays, H2AX phosphorylation (gamma-H2AX), and scoring of chromosome aberrations indicate that active RRMs produce DNA double-strand breaks (DSB) and chromosomal lesions in NB4, an acute myeloid leukemia (AML) cell line characterized by high sensitivity to RRMs. There is a direct quantitative correlation between the levels of DSBs and the cytotoxic/antiproliferative effects induced by RRMs. NB4.437r blasts, which are selectively resistant to RRMs, do not show any sign of DNA damage after treatment with ST1926, CD437, and analogues. DNA damage is the major mechanism underlying the antileukemic activity of RRMs in NB4 and other AML cell lines. In accordance with the S-phase specificity of the cytotoxic and antiproliferative responses of AML cells to RRMs, increases in DSBs are maximal during the S phase of the cell cycle. Induction of DSBs precedes inhibition of DNA replication and is associated with rapid activation of ataxia telangectasia mutated, ataxia telangectasia RAD3-related, and DNA-dependent protein kinases with subsequent stimulation of the p38 mitogen-activated protein kinase. Inhibition of ataxia telangectasia mutated and DNA-dependent protein kinases reduces phosphorylation of H2AX. Cells defective for homologous recombination are particularly sensitive to ST1926, indicating that this process is important for the protection of cells from the RRM-dependent DNA damage and cytotoxicity.
Cancer research 2005 JUL
Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells.
Abstract
Abstract
Activation of the transcription factor, nuclear factor-kappaB (NF-kappaB), results in up-regulation of not only antiapoptotic genes but also proapoptotic genes, including death receptor 4 (DR4) and death receptor 5 (DR5). Therefore, NF-kappaB activation either suppresses or promotes apoptosis depending on the type of stimulus or cell context. We showed previously that the synthetic retinoid, 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437), effectively induces apoptosis particularly in androgen-independent prostate carcinoma cells. This effect was associated with the ability of CD437 to induce the expression of DR4 and DR5. In the present study, we examined the hypothesis that NF-kappaB activation plays a role in CD437-induced death receptor expression and apoptosis. Treatment of DU145 cells with CD437 resulted in a rapid decrease (textgreater or = 3 hours) of IkappaBalpha, which was accompanied by increased translocation of the NF-kappaB subunit p65 from the cytoplasm to the nucleus and increased NF-kappaB DNA-binding activity (textgreater or = 4 hours). The NF-kappaB inhibitor, helenalin, inhibited CD437-induced IkappaBalpha reduction and p65 nuclear translocation. Accordingly, it also abrogated CD437-induced up-regulation of DR4, activation of caspase-8 and caspase-3, and increased DNA fragmentation. Overexpression of an IkappaBalpha dominant-negative mutant blocked not only CD437-induced p65 nuclear translocation but also DR4 up-regulation, caspase activation, and DNA fragmentation. CD437 was unable to decrease IkappaBalpha protein levels and up-regulate DR4 expression in CD437-resistant DU145 cells. Moreover, knockdown of Fas-associated death domain, caspase-8, and DR4, respectively, suppressed CD437-induced apoptosis. Collectively, these results indicate that CD437 activates NF-kappaB via decreasing IkappaBalpha protein and thereby induces DR4 expression and subsequent apoptosis in DU145 cells.
Leukemia 2002 APR
Classical and novel retinoids: their targets in cancer therapy.
Abstract
Abstract
Retinoids are important mediators of cellular growth and differentiation. Retinoids modulate the growth of both normal and malignant cells through their binding to retinoid nuclear receptors and their subsequent activation. While retinoids have demonstrated therapeutic efficacy in the treatment of acute promyelocytic leukemia, their spectrum of activity remains limited. Other agents such as histone deacetylase inhibitors may significantly increase retinoid activity in a number of malignant cell types. The novel retinoids N-(4-hydroxyphenyl) retinamide (4-HPR) and 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437; AHPN) induce apoptosis in a wide variety of malignant cells. Their mechanism(s) of action remain unclear, although a number of potential targets have been identified. Whether the retinoid receptors are involved in 4-HPR and CD473/AHPN mediated apoptosis remains unclear. Both 4-HPR and CD437/AHPN display significant potential as therapeutic agents in the treatment of a number of premalignant and malignant conditions.
Molecular pharmacology 2000 SEP
Dual mechanisms of action of the retinoid CD437: nuclear retinoic acid receptor-mediated suppression of squamous differentiation and receptor-independent induction of apoptosis in UMSCC22B human head and neck squamous cell carcinoma cells.
Abstract
Abstract
The synthetic retinoid 6-[3-(adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437), which can bind to and activate the nuclear retinoic acid receptors beta and gamma (RARbeta/gamma), is a potent inducer of apoptosis in various cancer cell lines. However, this effect was reported to be independent of RARs. In this study, we compared and contrasted the potencies and mechanisms of action of CD437 and several other receptor-selective retinoids in induction of apoptosis and modulation of squamous differentiation in UMSCC22B human head and neck squamous cell carcinoma cell line. CD437 and the structurally related retinoid CD2325 exhibited almost equal potency in inducing apoptosis, whereas several other retinoids failed to induce apoptosis. The RAR-specific pan antagonist AGN193109 failed to suppress CD437-induced apoptosis, indicating that the induction of apoptosis by CD437 was RAR-independent. c-Fos expression was induced by CD437 and CD2325 that induced apoptosis in the cell line but not by other retinoids that failed to induce apoptosis, suggesting a role for c-Fos in CD437-induced apoptosis. At low concentration (0.01 microM), CD437 shared with several other receptor-selective retinoids the ability to suppress the mRNA levels of the squamous differentiation markers Spr1, involucrin, and cytokeratin 1. This effect of CD437 could be blocked by AGN193109. We conclude that CD437 can exert its effects in UMSCC22B human human head and neck squamous cell carcinoma cells by at least two mechanisms: RAR-mediated suppression of squamous differentiation and RAR-independent induction of apoptosis.