CAY10603
Epigenetic modifier; Inhibits histone deacetylase (HDAC) 6
概要
CAY10603 is a selective and potent inhibitor of histone deacetylase 6 (HDAC6; IC₅₀ = 0.002 nM; Kozikowski et al.). HDAC6 deacetylates lysine residues on the N-terminal part of histones. HDAC6 is also known to regulate heat shock protein 90 (hsp90) via hyperacetylation (Rao et al.).
CANCER RESEARCH
· Inhibits growth of several pancreatic cancer cell lines (IC₅₀ = 0.1 - 1 μM) and SCCOHT, an ovarian cancer cell line (Kozikowski et al.; Wang et al.).
CANCER RESEARCH
· Inhibits growth of several pancreatic cancer cell lines (IC₅₀ = 0.1 - 1 μM) and SCCOHT, an ovarian cancer cell line (Kozikowski et al.; Wang et al.).
Alternative Names
HDAC6 inhibitor
Cell Type
Cancer Cells and Cell Lines
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research
CAS Number
1045792-66-2
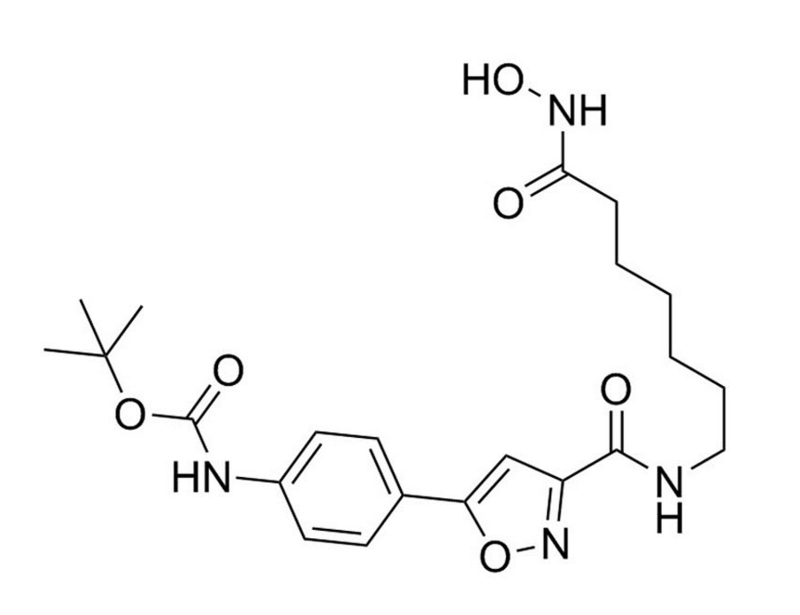
Chemical Formula
C₂₂H₃₀N₄O₆
Molecular Weight
446.5 g/mol
Purity
≥ 95%
Pathway
Epigenetic
Target
HDAC
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CAY10603 | 73582 | All | English |
| Safety Data Sheet | CAY10603 | 73582 | All | English |
数据及文献
Publications (2)
Blood 2008 SEP
HDAC6 inhibition enhances 17-AAG--mediated abrogation of hsp90 chaperone function in human leukemia cells.
Abstract
Abstract
Histone deacetylase 6 (HDAC6) is a heat shock protein 90 (hsp90) deacetylase. Treatment with pan-HDAC inhibitors or depletion of HDAC6 by siRNA induces hyperacetylation and inhibits ATP binding and chaperone function of hsp90. Treatment with 17-allylamino-demothoxy geldanamycin (17-AAG) also inhibits ATP binding and chaperone function of hsp90, resulting in polyubiquitylation and proteasomal degradation of hsp90 client proteins. In this study, we determined the effect of hsp90 hyperacetylation on the anti-hsp90 and antileukemia activity of 17-AAG. Hyperacetylation of hsp90 increased its binding to 17-AAG, as well as enhanced 17-AAG-mediated attenuation of ATP and the cochaperone p23 binding to hsp90. Notably, treatment with 17-AAG alone also reduced HDAC6 binding to hsp90 and induced hyperacetylation of hsp90. This promoted the proteasomal degradation of HDAC6. Cotreatment with 17-AAG and siRNA to HDAC6 induced more inhibition of hsp90 chaperone function and depletion of BCR-ABL and c-Raf than treatment with either agent alone. In addition, cotreatment with 17-AAG and tubacin augmented the loss of survival of K562 cells and viability of primary acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) samples. These findings demonstrate that HDAC6 is an hsp90 client protein and hyperacetylation of hsp90 augments the anti-hsp90 and antileukemia effects of 17-AAG.
Journal of medicinal chemistry 2008 AUG
Use of the nitrile oxide cycloaddition (NOC) reaction for molecular probe generation: a new class of enzyme selective histone deacetylase inhibitors (HDACIs) showing picomolar activity at HDAC6.
Abstract
Abstract
A series of hydroxamate based HDAC inhibitors containing a phenylisoxazole as the CAP group has been synthesized using nitrile oxide cycloaddition chemistry. An HDAC6 selective inhibitor having a potency of approximately 2 picomolar was identified. Some of the compounds were examined for their ability to block pancreatic cancer cell growth and found to be about 10-fold more potent than SAHA. This research provides valuable, new molecular probes for use in exploring HDAC biology.