BIRB-796
p38 MAPK inhibitor
概要
BIRB-796 is a highly potent inhibitor of p38 MAPK (Kd = 0.1 nM) that blocks TNFα release in LPS-stimulated THP-1 cells (IC₅₀ = 18 nM) (Pargellis et al.). At 10 μM, BIRB-796 can also inhibit JNK2α2 in vitro, but at lower concentrations BIRB-796 inhibits p38 MAPK without affecting phosphorylation of JNK substrates (Bain et al.; Kuma et al.).
MAINTENANCE AND SELF-RENEWAL
· Rescues the self-renewal ability of muscle satellite cells from aged mice (Bernet et al.).
· Increases the regenerative capacity of functional aged skeletal muscle stem cells grown in hydrogel (Cosgrove et al.).
· Blocks GADD45G-induced differentiation of long-term repopulating hematopoietic stem cells (Thalheimer et al.).
· Enhances stem cell activity of cultured umbilical cord blood-derived hematopoietic cells cultured in serum-free medium supplemented with SCF, TPO, and FLT3L (Baudet et al.).
· Blocks the osteogenic differentiation effect of myocilin on human mesenchymal stem cells (Kwon et al.).
MAINTENANCE AND SELF-RENEWAL
· Rescues the self-renewal ability of muscle satellite cells from aged mice (Bernet et al.).
· Increases the regenerative capacity of functional aged skeletal muscle stem cells grown in hydrogel (Cosgrove et al.).
· Blocks GADD45G-induced differentiation of long-term repopulating hematopoietic stem cells (Thalheimer et al.).
· Enhances stem cell activity of cultured umbilical cord blood-derived hematopoietic cells cultured in serum-free medium supplemented with SCF, TPO, and FLT3L (Baudet et al.).
· Blocks the osteogenic differentiation effect of myocilin on human mesenchymal stem cells (Kwon et al.).
Alternative Names
Doramapimod
Cell Type
Hematopoietic Stem and Progenitor Cells, Mesenchymal Stem and Progenitor Cells, Myogenic Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Expansion, Maintenance
Area of Interest
Stem Cell Biology
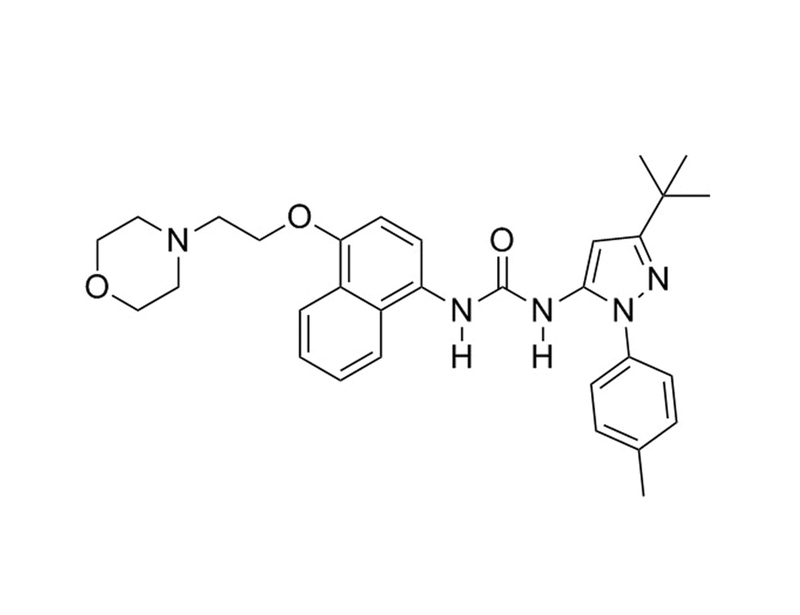
CAS Number
285983-48-4
Chemical Formula
C₃₁H₃₇N₅O₃
Molecular Weight
527.7 g/mol
Purity
≥ 98%
Pathway
p38 MAPK
Target
p38 MAPK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | BIRB-796 | 72682, 72684 | All | English |
| Safety Data Sheet | BIRB-796 | 72682, 72684 | All | English |
数据及文献
Publications (8)
Nature medicine 2014 MAR
p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice.
Abstract
Abstract
Skeletal muscle aging results in a gradual loss of skeletal muscle mass, skeletal muscle function and regenerative capacity, which can lead to sarcopenia and increased mortality. Although the mechanisms underlying sarcopenia remain unclear, the skeletal muscle stem cell, or satellite cell, is required for muscle regeneration. Therefore, identification of signaling pathways affecting satellite cell function during aging may provide insights into therapeutic targets for combating sarcopenia. Here, we show that a cell-autonomous loss in self-renewal occurs via alterations in fibroblast growth factor receptor-1, p38α and p38β mitogen-activated protein kinase signaling in satellite cells from aged mice. We further demonstrate that pharmacological manipulation of these pathways can ameliorate age-associated self-renewal defects. Thus, our data highlight an age-associated deregulation of a satellite cell homeostatic network and reveal potential therapeutic opportunities for the treatment of progressive muscle wasting.
Nature medicine 2014 MAR
Rejuvenation of the muscle stem cell population restores strength to injured aged muscles.
Abstract
Abstract
The elderly often suffer from progressive muscle weakness and regenerative failure. We demonstrate that muscle regeneration is impaired with aging owing in part to a cell-autonomous functional decline in skeletal muscle stem cells (MuSCs). Two-thirds of MuSCs from aged mice are intrinsically defective relative to MuSCs from young mice, with reduced capacity to repair myofibers and repopulate the stem cell reservoir in vivo following transplantation. This deficiency is correlated with a higher incidence of cells that express senescence markers and is due to elevated activity of the p38α and p38β mitogen-activated kinase pathway. We show that these limitations cannot be overcome by transplantation into the microenvironment of young recipient muscles. In contrast, subjecting the MuSC population from aged mice to transient inhibition of p38α and p38β in conjunction with culture on soft hydrogel substrates rapidly expands the residual functional MuSC population from aged mice, rejuvenating its potential for regeneration and serial transplantation as well as strengthening of damaged muscles of aged mice. These findings reveal a synergy between biophysical and biochemical cues that provides a paradigm for a localized autologous muscle stem cell therapy for the elderly.
Stem cell reports 2014 JUL
Cytokine-regulated GADD45G induces differentiation and lineage selection in hematopoietic stem cells.
Abstract
Abstract
The balance of self-renewal and differentiation in long-term repopulating hematopoietic stem cells (LT-HSC) must be strictly controlled to maintain blood homeostasis and to prevent leukemogenesis. Hematopoietic cytokines can induce differentiation in LT-HSCs; however, the molecular mechanism orchestrating this delicate balance requires further elucidation. We identified the tumor suppressor GADD45G as an instructor of LT-HSC differentiation under the control of differentiation-promoting cytokine receptor signaling. GADD45G immediately induces and accelerates differentiation in LT-HSCs and overrides the self-renewal program by specifically activating MAP3K4-mediated MAPK p38. Conversely, the absence of GADD45G enhances the self-renewal potential of LT-HSCs. Videomicroscopy-based tracking of single LT-HSCs revealed that, once GADD45G is expressed, the development of LT-HSCs into lineage-committed progeny occurred within 36 hr and uncovered a selective lineage choice with a severe reduction in megakaryocytic-erythroid cells. Here, we report an unrecognized role of GADD45G as a central molecular linker of extrinsic cytokine differentiation and lineage choice control in hematopoiesis.
The Journal of biological chemistry 2013 JUN
Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling.
Abstract
Abstract
Myocilin is a secreted glycoprotein that is expressed in ocular and non-ocular tissues. Mutations in the MYOCILIN gene may lead to juvenile- and adult-onset primary open-angle glaucoma. Here we report that myocilin is expressed in bone marrow-derived mesenchymal stem cells (MSCs) and plays a role in their differentiation into osteoblasts in vitro and in osteogenesis in vivo. Expression of myocilin was detected in MSCs derived from mouse, rat, and human bone marrow, with human MSCs exhibiting the highest level of myocilin expression. Expression of myocilin rose during the course of human MSC differentiation into osteoblasts but not into adipocytes, and treatment with exogenous myocilin further enhanced osteogenesis. MSCs derived from Myoc-null mice had a reduced ability to differentiate into the osteoblastic lineage, which was partially rescued by exogenous extracellular myocilin treatment. Myocilin also stimulated osteogenic differentiation of wild-type MSCs, which was associated with activation of the p38, Erk1/2, and JNK MAP kinase signaling pathways as well as up-regulated expression of the osteogenic transcription factors Runx2 and Dlx5. Finally, cortical bone thickness and trabecular volume, as well as the expression level of osteopontin, a known factor of bone remodeling and osteoblast differentiation, were reduced dramatically in the femurs of Myoc-null mice compared with wild-type mice. These data suggest that myocilin should be considered as a target for improving the bone regenerative potential of MSCs and may identify a new role for myocilin in bone formation and/or maintenance in vivo.
Blood 2012 JUN
RNAi screen identifies MAPK14 as a druggable suppressor of human hematopoietic stem cell expansion.
Abstract
Abstract
We report on a forward RNAi screen in primary human hematopoietic stem and progenitor cells, using pooled lentiviral shRNA libraries deconvoluted by next generation sequencing. We identify MAPK14/p38α as a modulator of ex vivo stem cell proliferation and show that pharmacologic inhibition of p38 dramatically enhances the stem cell activity of cultured umbilical cord blood derived hematopoietic cells. p38 inhibitors should thus be considered in strategies aiming at expanding stem cells for clinical benefit.
The Biochemical journal 2007 DEC
The selectivity of protein kinase inhibitors: a further update.
Abstract
Abstract
The specificities of 65 compounds reported to be relatively specific inhibitors of protein kinases have been profiled against a panel of 70-80 protein kinases. On the basis of this information, the effects of compounds that we have studied in cells and other data in the literature, we recommend the use of the following small-molecule inhibitors: SB 203580/SB202190 and BIRB 0796 to be used in parallel to assess the physiological roles of p38 MAPK (mitogen-activated protein kinase) isoforms, PI-103 and wortmannin to be used in parallel to inhibit phosphatidylinositol (phosphoinositide) 3-kinases, PP1 or PP2 to be used in parallel with Src-I1 (Src inhibitor-1) to inhibit Src family members; PD 184352 or PD 0325901 to inhibit MKK1 (MAPK kinase-1) or MKK1 plus MKK5, Akt-I-1/2 to inhibit the activation of PKB (protein kinase B/Akt), rapamycin to inhibit TORC1 [mTOR (mammalian target of rapamycin)-raptor (regulatory associated protein of mTOR) complex], CT 99021 to inhibit GSK3 (glycogen synthase kinase 3), BI-D1870 and SL0101 or FMK (fluoromethylketone) to be used in parallel to inhibit RSK (ribosomal S6 kinase), D4476 to inhibit CK1 (casein kinase 1), VX680 to inhibit Aurora kinases, and roscovitine as a pan-CDK (cyclin-dependent kinase) inhibitor. We have also identified harmine as a potent and specific inhibitor of DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A) in vitro. The results have further emphasized the need for considerable caution in using small-molecule inhibitors of protein kinases to assess the physiological roles of these enzymes. Despite being used widely, many of the compounds that we analysed were too non-specific for useful conclusions to be made, other than to exclude the involvement of particular protein kinases in cellular processes.