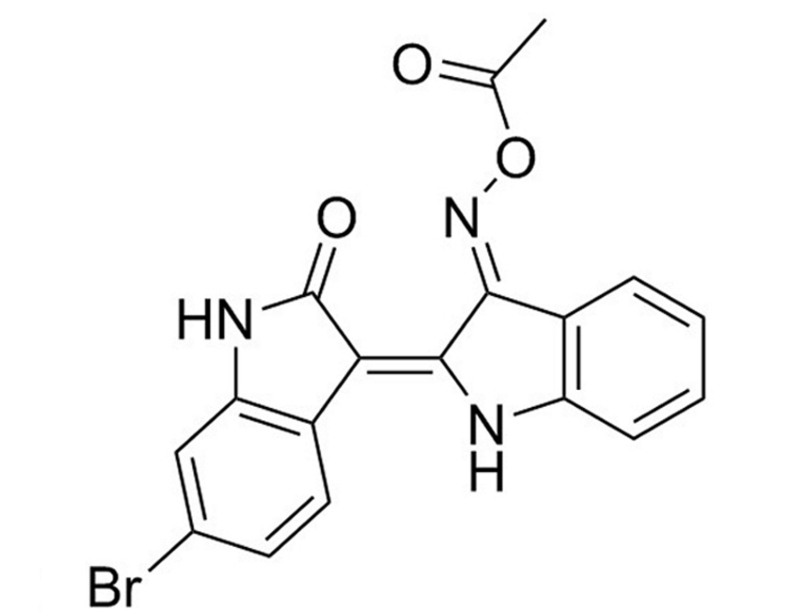
BIO-Acetoxime
WNT pathway activator; Inhibits GSK3
概要
BIO-Acetoxime is an analog of the GSK3 inhibitor BIO (Catalog #72032). It potently inhibits GSK3α and GSK3β (IC₅₀ = 10 nM), and less potently inhibits cyclin-dependent kinases Cdk5/p25, Cdk2/A, and Cdk1/B (IC₅₀ = 2.4, 4.3, and 63 µM, respectively; Meijer et al.; Polychronopoulos et al.). By inhibiting GSK3β, BIO-Acetoxime blocks β-catenin phosphorylation and degradation, thereby allowing it to activate transcription of WNT pathway-controlled genes (Meijer et al.).
IMMUNOLOGY
· Inhibits CD8+ effector T cell differentiation (Zhou et al.).
· Suppresses viral gene expression and protects oral epithelial cells from herpes simplex virus-1 infection (Hsu & Hung).
IMMUNOLOGY
· Inhibits CD8+ effector T cell differentiation (Zhou et al.).
· Suppresses viral gene expression and protects oral epithelial cells from herpes simplex virus-1 infection (Hsu & Hung).
Alternative Names
6-Bromoindirubin-3’-acetoxime; BIA; GSK-3 inhibitor X
Cell Type
T Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Immunology
CAS Number
740841-15-0
Chemical Formula
C₁₈H₁₂BrN₃O₃
Molecular Weight
398.2 g/mol
Purity
≥ 95%
Pathway
WNT
Target
GSK3
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | BIO-Acetoxime | 73322 | All | English |
| Safety Data Sheet | BIO-Acetoxime | 73322 | All | English |
数据及文献
Publications (4)
Archives of virology 2013 JUN
Antiherpetic potential of 6-bromoindirubin-3'-acetoxime (BIO-acetoxime) in human oral epithelial cells.
Abstract
Abstract
Glycogen synthase kinase 3 (GSK-3) functions in the regulation of glycogen metabolism, in the cell cycle, and in immune responses and is targeted by some viruses to favor the viral life cycle. Inhibition of GSK-3 by 6-bromoindirubin-3'-acetoxime (BIO-acetoxime), a synthetic derivative of a compound from the Mediterranean mollusk Hexaplex trunculus, protects cells from varicella infection. In this study, we examined the effects of BIO-acetoxime against herpes simplex virus-1 (HSV-1) infection in human oral epithelial cells, which represent a natural target cell type. The results revealed that BIO-acetoxime relieves HSV-1-induced cytopathic effects and apoptosis. We also found that BIO-acetoxime reduced viral yields and the expression of different classes of viral proteins. Furthermore, addition of BIO-acetoxime before, simultaneously with or after HSV-1 infection significantly reduced viral yields. Collectively, BIO-acetoxime may suppress viral gene expression and protect oral epithelial cells from HSV-1 infection. These results suggest the possible involvement of GSK-3 in HSV-1 infection.
Immunity 2010 AUG
Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1.
Abstract
Abstract
T cell factor 1 (TCF-1) is a transcription factor known to act downstream of the canonical Wnt pathway and is essential for normal T cell development. However, its physiological roles in mature CD8(+) T cell responses are unknown. Here we showed that TCF-1 deficiency limited proliferation of CD8(+) effector T cells and impaired their differentiation toward a central memory phenotype. Moreover, TCF-1-deficient memory CD8(+) T cells were progressively lost over time, exhibiting reduced expression of the antiapoptotic molecule Bcl-2 and interleukin-2 receptor beta chain and diminished IL-15-driven proliferation. TCF-1 was directly associated with the Eomes allele and the Wnt-TCF-1 pathway was necessary and sufficient for optimal Eomes expression in naive and memory CD8(+) T cells. Importantly, forced expression of Eomes partly protected TCF-1-deficient memory CD8(+) T cells from time-dependent attrition. Our studies thus identify TCF-1 as a critical player in a transcriptional program that regulates memory CD8 differentiation and longevity.
Journal of medicinal chemistry 2004 FEB
Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases.
Abstract
Abstract
Pharmacological inhibitors of glycogen synthase kinase-3 (GSK-3) and cyclin-dependent kinases have a promising potential for applications against several neurodegenerative diseases such as Alzheimer's disease. Indirubins, a family of bis-indoles isolated from various natural sources, are potent inhibitors of several kinases, including GSK-3. Using the cocrystal structures of various indirubins with GSK-3beta, CDK2 and CDK5/p25, we have modeled the binding of indirubins within the ATP-binding pocket of these kinases. This modeling approach provided some insight into the molecular basis of indirubins' action and selectivity and allowed us to forecast some improvements of this family of bis-indoles as kinase inhibitors. Predicted molecules, including 6-substituted and 5,6-disubstituted indirubins, were synthesized and evaluated as CDK and GSK-3 inhibitors. Control, kinase-inactive indirubins were obtained by introduction of a methyl substitution on N1.
Chemistry & biology 2003 DEC
GSK-3-selective inhibitors derived from Tyrian purple indirubins.
Abstract
Abstract
Gastropod mollusks have been used for over 2500 years to produce the Tyrian purple" dye made famous by the Phoenicians. This dye is constituted of mixed bromine-substituted indigo and indirubin isomers. Among these�