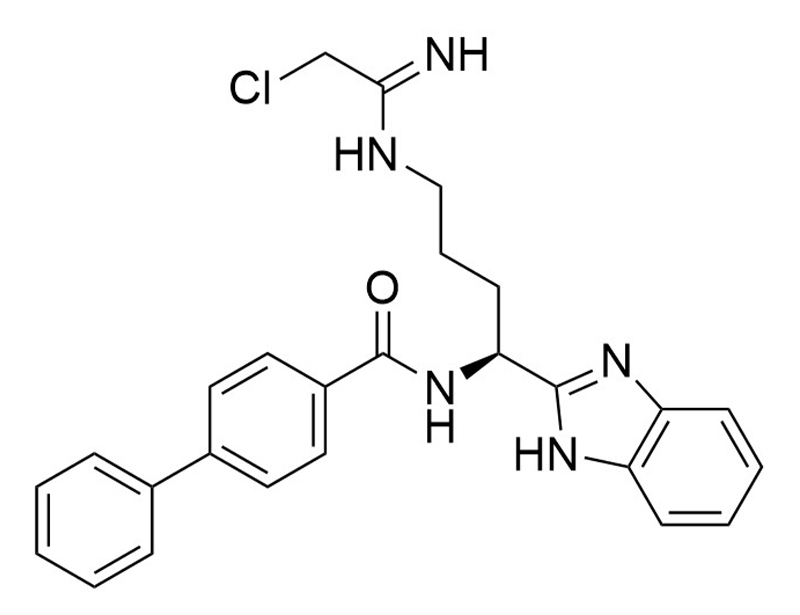
BB-Cl-Amidine
Inhibits protein-arginine deiminase (PAD)
概要
BB-Cl-Amidine is a protein-arginine deiminase (PAD) inhibitor that irreversibly inhibits four subtypes of PAD (kinact/Ki = 16,100/PAD1, 4,100/PAD2, 6,800/PAD3, and 13,300/PAD4 M-1min-1; Luo et al.; Muth et al.). BB-Cl-Amidine is less susceptible to proteolysis than Cl-amidine due to the addition of benzimidazole on its C-terminus (Knight et al.).
IMMUNOLOGY
· Inhibits neutrophil extracellular traps formation in neutrophils (Knight et al.).
IMMUNOLOGY
· Inhibits neutrophil extracellular traps formation in neutrophils (Knight et al.).
Cell Type
Cancer Cells and Cell Lines, Granulocytes and Subsets
Area of Interest
Cancer Research, Immunology
CAS Number
1802637-39-3
Chemical Formula
C26H26ClN5O
Molecular Weight
460 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | BB-Cl-Amidine | 100-0516, 100-0517 | All | English |
| Safety Data Sheet | BB-Cl-Amidine | 100-0516, 100-0517 | All | English |
数据及文献
Publications (3)
Journal of medicinal chemistry 2017
Development of a Selective Inhibitor of Protein Arginine Deiminase 2.
Abstract
Abstract
Protein arginine deiminase 2 (PAD2) plays a key role in the onset and progression of multiple sclerosis, rheumatoid arthritis, and breast cancer. To date, no PAD2-selective inhibitor has been developed. Such a compound will be critical for elucidating the biological roles of this isozyme and may ultimately be useful for treating specific diseases in which PAD2 activity is dysregulated. To achieve this goal, we synthesized a series of benzimidazole-based derivatives of Cl-amidine, hypothesizing that this scaffold would allow access to a series of PAD2-selective inhibitors with enhanced cellular efficacy. Herein, we demonstrate that substitutions at both the N-terminus and C-terminus of Cl-amidine result in {\textgreater}100-fold increases in PAD2 potency and selectivity (30a, 41a, and 49a) as well as cellular efficacy (30a). Notably, these compounds use the far less reactive fluoroacetamidine warhead. In total, we predict that 30a will be a critical tool for understanding cellular PAD2 function and sets the stage for treating diseases in which PAD2 activity is dysregulated.
Annals of the rheumatic diseases 2015 dec
Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice.
Abstract
Abstract
OBJECTIVES An imbalance between neutrophil extracellular trap (NET) formation and degradation has been described in systemic lupus erythematosus (SLE), potentially contributing to autoantigen externalisation, type I interferon synthesis and endothelial damage. We have demonstrated that peptidylarginine deiminase (PAD) inhibition reduces NET formation and protects against lupus-related vascular damage in the New Zealand Mixed model of lupus. However, another strategy for inhibiting NETs--knockout of NOX2--accelerates lupus in a different murine model, MRL/lpr. Here, we test the effects of PAD inhibition on MRL/lpr mice in order to clarify whether some NET inhibitory pathways may be consistently therapeutic across models of SLE. METHODS NET formation and autoantibodies to NETs were characterised in lupus-prone MRL/lpr mice. MRL/lpr mice were also treated with two different PAD inhibitors, Cl-amidine and the newly described BB-Cl-amidine. NET formation, endothelial function, interferon signature, nephritis and skin disease were examined in treated mice. RESULTS Neutrophils from MRL/lpr mice demonstrate accelerated NET formation compared with controls. MRL/lpr mice also form autoantibodies to NETs and have evidence of endothelial dysfunction. PAD inhibition markedly improves endothelial function, while downregulating the expression of type I interferon-regulated genes. PAD inhibition also reduces proteinuria and immune complex deposition in the kidneys, while protecting against skin disease. CONCLUSIONS PAD inhibition reduces NET formation, while protecting against lupus-related damage to the vasculature, kidneys and skin in various lupus models. The strategy by which NETs are inhibited will have to be carefully considered if human studies are to be undertaken.
Biochemistry 2006 oct
Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization.
Abstract
Abstract
Protein arginine deiminase 4 (PAD4) is a transcriptional coregulator that catalyzes the calcium-dependent conversion of specific arginine residues in proteins to citrulline. Recently, we reported the synthesis and characterization of F-amidine, a potent and bioavailable irreversible inactivator of PAD4. Herein, we report our efforts to identify the steric and leaving group requirements for F-amidine-induced PAD4 inactivation, the structure of the PAD4-F-amidine x calcium complex, and in vivo studies with N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide (Cl-amidine), a PAD4 inactivator with enhanced potency. The PAD4 inactivators described herein will be useful pharmacological probes in characterizing the incompletely defined physiological role(s) of this enzyme. In addition, they represent potential lead compounds for the treatment of rheumatoid arthritis because a growing body of evidence supports a role for PAD4 in the onset and progression of this chronic autoimmune disorder.