All-Trans Retinoic Acid
Retinoid pathway activator; Activates retinoic acid receptor (RAR)
概要
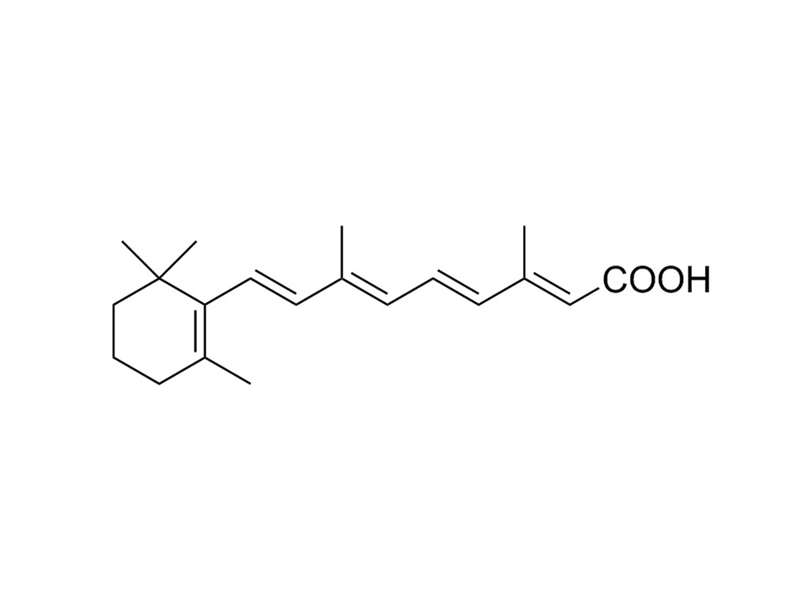
All-Trans Retinoic Acid is a derivative of Vitamin A that functions as a ligand for the retinoic acid receptor (RAR, IC₅₀ = 14 nM). RARs heterodimerize with retinoid X receptors (RXRs) and bind to retinoic acid response elements (RAREs) in DNA and act as transcription factors, altering gene expression. (Apfel et al., Chambon)
DIFFERENTIATION
· Promotes differentiation of motor neurons from mouse and human pluripotent stem cells (Dimos et al., Wichterle et al.).
· Promotes differentiation of neurons from neural stem cells (Takahashi et al.).
· Promotes differentiation of pancreatic progenitors from human embryonic stem (ES) cells (D'Amour et al.).
· Promotes differentiation of adipocytes from mouse ES cells (Dani et al.).
· Promotes differentiation of ventricular cardiomyocytes from mouse ES cells (Wobus et al.).
· Promotes terminal differentiation of granulocytes (Collins).
CANCER RESEARCH
· Promotes maturation of blast cells in differentiation therapy of acute promyelocytic leukemia (Huang et al.).
DIFFERENTIATION
· Promotes differentiation of motor neurons from mouse and human pluripotent stem cells (Dimos et al., Wichterle et al.).
· Promotes differentiation of neurons from neural stem cells (Takahashi et al.).
· Promotes differentiation of pancreatic progenitors from human embryonic stem (ES) cells (D'Amour et al.).
· Promotes differentiation of adipocytes from mouse ES cells (Dani et al.).
· Promotes differentiation of ventricular cardiomyocytes from mouse ES cells (Wobus et al.).
· Promotes terminal differentiation of granulocytes (Collins).
CANCER RESEARCH
· Promotes maturation of blast cells in differentiation therapy of acute promyelocytic leukemia (Huang et al.).
Alternative Names
ATRA; NSC 122758; Retinoic acid; Trans retinoic acid; Tretinoin; Vitamin A acid
Cell Type
Adipocytes, Cardiomyocytes, PSC-Derived, Endoderm, PSC-Derived, Granulocytes and Subsets, Leukemia/Lymphoma Cells, Mesoderm, PSC-Derived, Neural Cells, PSC-Derived, Neurons, Pancreatic Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Cancer Research, Neuroscience, Stem Cell Biology
CAS Number
302-79-4
Chemical Formula
C₂₀H₂₈O₂
Molecular Weight
300.4 g/mol
Purity
≥ 98%
Pathway
Retinoid
Target
RAR
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | All-Trans Retinoic Acid | 72262, 72264 | All | English |
| Product Information Sheet 2 | All-Trans Retinoic Acid | 72262, 72264 | All | English |
| Safety Data Sheet | All-Trans Retinoic Acid | 72262, 72264 | All | English |
数据及文献
Publications (10)
Science (New York, N.Y.) 2008 AUG
Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons.
Abstract
Abstract
The generation of pluripotent stem cells from an individual patient would enable the large-scale production of the cell types affected by that patient's disease. These cells could in turn be used for disease modeling, drug discovery, and eventually autologous cell replacement therapies. Although recent studies have demonstrated the reprogramming of human fibroblasts to a pluripotent state, it remains unclear whether these induced pluripotent stem (iPS) cells can be produced directly from elderly patients with chronic disease. We have generated iPS cells from an 82-year-old woman diagnosed with a familial form of amyotrophic lateral sclerosis (ALS). These patient-specific iPS cells possess properties of embryonic stem cells and were successfully directed to differentiate into motor neurons, the cell type destroyed in ALS.
Nature biotechnology 2006 NOV
Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells.
Abstract
Abstract
Of paramount importance for the development of cell therapies to treat diabetes is the production of sufficient numbers of pancreatic endocrine cells that function similarly to primary islets. We have developed a differentiation process that converts human embryonic stem (hES) cells to endocrine cells capable of synthesizing the pancreatic hormones insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin. This process mimics in vivo pancreatic organogenesis by directing cells through stages resembling definitive endoderm, gut-tube endoderm, pancreatic endoderm and endocrine precursor--en route to cells that express endocrine hormones. The hES cell-derived insulin-expressing cells have an insulin content approaching that of adult islets. Similar to fetal beta-cells, they release C-peptide in response to multiple secretory stimuli, but only minimally to glucose. Production of these hES cell-derived endocrine cells may represent a critical step in the development of a renewable source of cells for diabetes cell therapy.
Leukemia 2002 OCT
The role of retinoids and retinoic acid receptors in normal hematopoiesis.
Abstract
Abstract
The dramatic therapeutic activity of all-trans retinoic acid (ATRA) in inducing terminal granulocytic differentiation of the malignant promyelocytes that characterize human acute promyelocytic leukemia (APL) has led to numerous studies assessing the role of retinoids and the retinoic acid receptors (RARs) in the regulation of normal hematopoiesis. Studies with knock out mice indicate that retinoic acid receptor activity is not essential for normal hematopoiesis, but both in vitro and in vivo studies indicate that these receptors may be important modifiers/regulators of different myeloid precursors/ progenitors including the primitive transplantable stem cell. A number of target genes have been identified that are either directly or indirectly regulated by RA receptors and which likely play important roles in the retinoid-mediated regulation of myelopoiesis. Several in vitro models of hematopoiesis suggest that the transcriptional activity of RA receptors is developmentally regulated during different stages of myelopoiesis. This regulation might involve non-ligand mediated molecular events that alter the interaction of RA receptors with transcriptional corepressor complexes. Moreover, the interaction of RA receptors with other families of transcription factors expressed in different hematopoietic lineages might also account for differential RA receptor activity at different stages of myelopoiesis.
Cell 2002 AUG
Directed differentiation of embryonic stem cells into motor neurons.
Abstract
Abstract
Inductive signals and transcription factors involved in motor neuron generation have been identified, raising the question of whether these developmental insights can be used to direct stem cells to a motor neuron fate. We show that developmentally relevant signaling factors can induce mouse embryonic stem (ES) cells to differentiate into spinal progenitor cells, and subsequently into motor neurons, through a pathway recapitulating that used in vivo. ES cell-derived motor neurons can populate the embryonic spinal cord, extend axons, and form synapses with target muscles. Thus, inductive signals involved in normal pathways of neurogenesis can direct ES cells to form specific classes of CNS neurons.
Journal of neurobiology 1999 JAN
Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures.
Abstract
Abstract
The adult rat hippocampus contains fibroblast growth factor 2-responsive stem cells that are self-renewing and have the ability to generate both neurons and glia in vitro, but little is known about the molecular events that regulate stem cell differentiation. Hippocampus-derived stem cell clones were used to examine the effects of retinoic acid (RA) on neuronal differentiation. Exposure to RA caused an immediate up-regulation of NeuroD, increased p21 expression, and concurrent exit from cell cycle. These changes were accompanied by a threefold increase in the number of cells differentiating into immature neurons. An accompanying effect of RA was to sustain or up-regulate trkA, trkB, trkC, and p75NGFR expression. Without RA treatment, cells were minimally responsive to neurotrophins (NTs), whereas the sequential application of RA followed by brain-derived neurotrophic factor or NT-3 led to a significant increase in neurons displaying mature y-a-minobutyric acid, acetylcholinesterase, tyrosine hydroxylase, or calbindin phenotypes. Although NTs promoted maturation, they had little effect on the total number of neurons generated, suggesting that RA and neurotrophins acted at distinct stages in neurogenesis. RA first promoted the acquisition of a neuronal fate, and NTs subsequently enhanced maturation by way of RA-dependent expression of the Trk receptors. In combination, these sequential effects were sufficient to stimulate stem cell-derived progenitors to differentiate into neurons displaying a variety of transmitter phenotypes.
Journal of cell science 1997 JUN
Differentiation of embryonic stem cells into adipocytes in vitro.
Abstract
Abstract
Embryonic stem cells, derived from the inner cell mass of murine blastocysts, can be maintained in a totipotent state in vitro. In appropriate conditions embryonic stem cells have been shown to differentiate in vitro into various derivatives of all three primary germ layers. We describe in this paper conditions to induce differentiation of embryonic stem cells reliably and at high efficiency into adipocytes. A prerequisite is to treat early developing embryonic stem cell-derived embryoid bodies with retinoic acid for a precise period of time. Retinoic acid could not be substituted by adipogenic hormones nor by potent activators of peroxisome proliferator-activated receptors. Treatment with retinoic acid resulted in the subsequent appearance of large clusters of mature adipocytes in embryoid body outgrowths. Lipogenic and lipolytic activities as well as high level expression of adipocyte specific genes could be detected in these cultures. Analysis of expression of potential adipogenic genes, such as peroxisome proliferator-activated receptors gamma and delta and CCAAT/enhancer binding protein beta, during differentiation of retinoic acid-treated embryoid bodies has been performed. The temporal pattern of expression of genes encoding these nuclear factors resembled that found during mouse embryogenesis. The differentiation of embryonic stem cells into adipocytes will provide an invaluable model for the characterisation of the role of genes expressed during the adipocyte development programme and for the identification of new adipogenic regulatory genes.