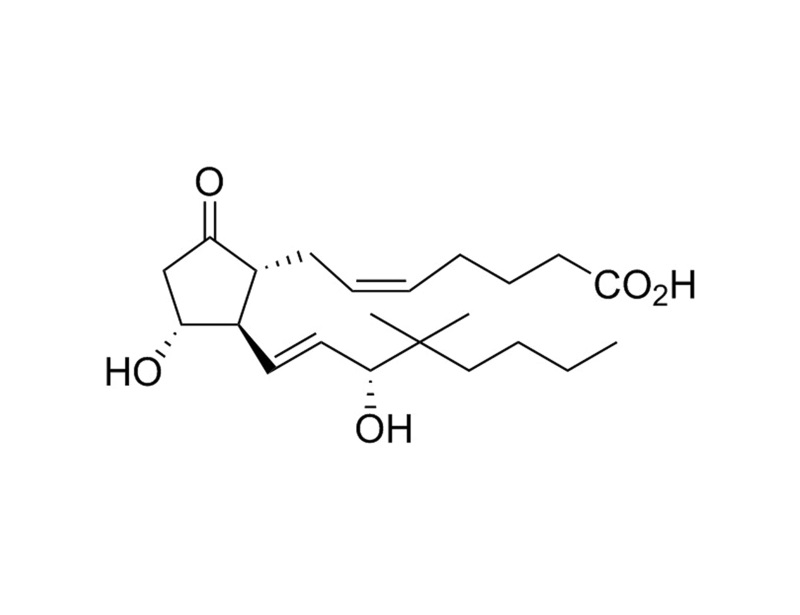
16,16-Dimethyl Prostaglandin E2
Prostanoid pathway activator; Inhibits 15-hydroxy PGDH
概要
16,16-dimethyl Prostaglandin E2 (16,16-dimethyl PGE2) is a competitive inhibitor of 15-hydroxy PGDH (North et al.; Ohno et al). 16,16-dimethyl PGE2 acts as an agonist on most prostaglandin E (EP) receptor subtypes (Coleman et al.; Robert et al.) with a Kd for activation of about 1 nM (Coleman et al.). 16,16-dimethyl PGE2 is supplied in methyl acetate solution at 10 mg/mL (26 mM).
MAINTENANCE AND SELF-RENEWAL
· Increased hematopoietic stem and progenitor cell (HSPC) numbers in zebrafish aorta-gonad-mesonephros (AGM) region and mouse bone marrow (North et al.).
· Mediates the effects of WNT on zebrafish HSPC self-renewal (Goessling et al.).
MAINTENANCE AND SELF-RENEWAL
· Increased hematopoietic stem and progenitor cell (HSPC) numbers in zebrafish aorta-gonad-mesonephros (AGM) region and mouse bone marrow (North et al.).
· Mediates the effects of WNT on zebrafish HSPC self-renewal (Goessling et al.).
Alternative Names
16,16-dimethyl PGE2
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Expansion
Area of Interest
Stem Cell Biology
CAS Number
39746-25-3
Chemical Formula
C₂₂H₃₆O₅
Molecular Weight
380.5 g/mol
Purity
≥ 95%
Pathway
Prostanoid
Target
15-hydroxy PGDH
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | 16,16-Dimethyl Prostaglandin E2 | 72372 | All | English |
| Safety Data Sheet | 16,16-Dimethyl Prostaglandin E2 | 72372 | All | English |
数据及文献
Publications (5)
Cell 2009 MAR
Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration.
Abstract
Abstract
Interactions between developmental signaling pathways govern the formation and function of stem cells. Prostaglandin (PG) E2 regulates vertebrate hematopoietic stem cells (HSC). Similarly, the Wnt signaling pathway controls HSC self-renewal and bone marrow repopulation. Here, we show that wnt reporter activity in zebrafish HSCs is responsive to PGE2 modulation, demonstrating a direct interaction in vivo. Inhibition of PGE2 synthesis blocked wnt-induced alterations in HSC formation. PGE2 modified the wnt signaling cascade at the level of beta-catenin degradation through cAMP/PKA-mediated stabilizing phosphorylation events. The PGE2/Wnt interaction regulated murine stem and progenitor populations in vitro in hematopoietic ES cell assays and in vivo following transplantation. The relationship between PGE2 and Wnt was also conserved during regeneration of other organ systems. Our work provides in vivo evidence that Wnt activation in stem cells requires PGE2, and suggests the PGE2/Wnt interaction is a master regulator of vertebrate regeneration and recovery.
Nature 2007 JUN
Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis.
Abstract
Abstract
Haematopoietic stem cell (HSC) homeostasis is tightly controlled by growth factors, signalling molecules and transcription factors. Definitive HSCs derived during embryogenesis in the aorta-gonad-mesonephros region subsequently colonize fetal and adult haematopoietic organs. To identify new modulators of HSC formation and homeostasis, a panel of biologically active compounds was screened for effects on stem cell induction in the zebrafish aorta-gonad-mesonephros region. Here, we show that chemicals that enhance prostaglandin (PG) E2 synthesis increased HSC numbers, and those that block prostaglandin synthesis decreased stem cell numbers. The cyclooxygenases responsible for PGE2 synthesis were required for HSC formation. A stable derivative of PGE2 improved kidney marrow recovery following irradiation injury in the adult zebrafish. In murine embryonic stem cell differentiation assays, PGE2 caused amplification of multipotent progenitors. Furthermore, ex vivo exposure to stabilized PGE2 enhanced spleen colony forming units at day 12 post transplant and increased the frequency of long-term repopulating HSCs present in murine bone marrow after limiting dilution competitive transplantation. The conserved role for PGE2 in the regulation of vertebrate HSC homeostasis indicates that modulation of the prostaglandin pathway may facilitate expansion of HSC number for therapeutic purposes.
Pharmacological reviews 1994 JUN
International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes.
Abstract
Abstract
Journal of biochemistry 1978 DEC
Studies on 15-hydroxyprostaglandin dehydrogenase with various prostaglandin analogues.
Abstract
Abstract
The NAD+-linked 15-hydroxyprostaglandin dehydrogenase (PGDH) of swine lung was purified to a high specific activity by affinity chromatographies on prostaglandin (PG)-and NAD+-Sepharose. The affinities of the enzyme for various synthetic analogues of PGA, E, F, and I and their inhibitory effects on the enzymatic reaction were examined. The modification of the alkyl side chain of PG, particularly at C-15 or C-16, reduced the affinity of the enzyme for these PG analogues. Furthermore, 14-methyl-13,14-dihydro-PGE1 and 16-cyclopentyl-omega-trinor-15-epi-PGE2 were potent inhibitors of PGDH.
Gastroenterology 1976 MAR
Gastric antisecretory and antiulcer properties of PGE2, 15-methyl PGE2, and 16, 16-dimethyl PGE2. Intravenous, oral and intrajejunal administration.
Abstract
Abstract
15-Methyl PGE2 and 16,16-dimethyl PGE2 were found (1) to be 40 and 100 times, respectively, more potent than PGE2 after intravenous administration in inhibiting histamine-stimulated gastric secretion in dogs with a denervated (Heidenhain) gastric pouch, (2) to be active orally and intrajejunally, whereas PGE2 was inactive, and (3) to exert antisecretory activity for longer duration than PGE2. 16,16-Dimethyl PGE2 was about 2.5 times more potent than 15-methyl PGE2. Volume, acid concentration, and output, and pepsin output (but not concentration) were reduced in a dose-dependent manner. In the rat, 16,16-dimethyl PGE2 also inhibited gastric secretion and prevented the formation of ulcers produced by various methods: gastric ulcers (Shay, and steroid induced) and duodenal ulcers (secretogogue induced). In this species, 1l816-dimethyl PGE2 was 2 to 50 times more potent than PGE2, depending on the endpoint, and was active orally. These prostaglandins appear to inhibit gastric acid secretion by acting directly on the parietal cells, and making these unresponsive to most stimulants. Vomiting was a side effect of the prostaglandin analogues in the dog, but almost exclusively when these were given orally. After intravenous or intrajejunal administration at doses inhibiting gastric secretion by 80%, vomiting was seen only once. These results suggest that 15-methyl PGE2 and 16,16-dimethyl PGE2 may be of value in the treatment of peptic ulcer.