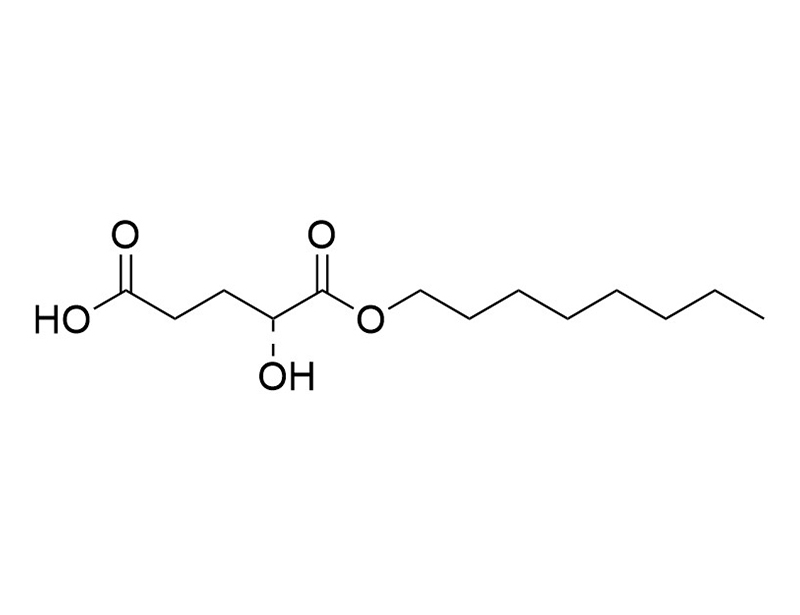
(2R)-Octyl-α-hydroxyglutarate
Inhibits α-ketoglutarate-dependent dioxygenases
概要
(2R)-Octyl-α-hydroxyglutarate is a cell-permeable derivative of α-hydroxyglutarate (2-HG). D- and L-2-hydroxyglutarate dehydrogenases convert 2-HG to α-ketoglutarate (2-oxoglutarate), which, along with oxygen, are co-substrates for a large family of αKG-dependent dioxygenases. 2-HG itself inhibits αKG-dependent dioxygenases by competing with α-ketoglutarate for binding to this family of enzymes (Xu et al.). Mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2 are associated with certain cancers (Reitman et al.; Sulkowski et al.). These enzymes convert isocitrate to α-hydroxyglutarate (2-HG) rather than α-ketoglutarate, and this results in high levels of accumulated 2-HG in cancer cells. (2R)-Octyl-α-hydroxyglutarate is useful for investigating oxidative mitochondrial processes of IDH mutations in cancer.
CANCER RESEARCH
· Inhibits α-ketoglutarate (αKG)-dependent dioxygenases (Sulkowski et al.; Xu et al.).
CANCER RESEARCH
· Inhibits α-ketoglutarate (αKG)-dependent dioxygenases (Sulkowski et al.; Xu et al.).
Alternative Names
(2R)-Octyl-2-HG
Area of Interest
Cancer Research
CAS Number
1391194-67-4
Chemical Formula
C13H24O5
Molecular Weight
260.3 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | (2R)-Octyl-α-hydroxyglutarate | 100-0514, 100-0515 | All | English |
| Safety Data Sheet | (2R)-Octyl-α-hydroxyglutarate | 100-0514, 100-0515 | All | English |
数据及文献
Publications (3)
Science translational medicine 2017
2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity.
Abstract
Abstract
2-Hydroxyglutarate (2HG) exists as two enantiomers, (R)-2HG and (S)-2HG, and both are implicated in tumor progression via their inhibitory effects on $\alpha$-ketoglutarate ($\alpha$KG)-dependent dioxygenases. The former is an oncometabolite that is induced by the neomorphic activity conferred by isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations, whereas the latter is produced under pathologic processes such as hypoxia. We report that IDH1/2 mutations induce a homologous recombination (HR) defect that renders tumor cells exquisitely sensitive to poly(adenosine 5'-diphosphate-ribose) polymerase (PARP) inhibitors. This BRCAness" phenotype of IDH mutant cells can be completely reversed by treatment with small-molecule inhibitors of the mutant IDH1 enzyme and conversely it can be entirely recapitulated by treatment with either of the 2HG enantiomers in cells with intact IDH1/2 proteins. We demonstrate mutant IDH1-dependent PARP inhibitor sensitivity in a range of clinically relevant models including primary patient-derived glioma cells in culture and genetically matched tumor xenografts in vivo. These findings provide the basis for a possible therapeutic strategy exploiting the biological consequences of mutant IDH rather than attempting to block 2HG production by targeting the 2HG-dependent HR deficiency with PARP inhibition. Furthermore our results uncover an unexpected link between oncometabolites altered DNA repair and genetic instability."
The Journal of biological chemistry 2014 aug
Cancer-associated isocitrate dehydrogenase 1 (IDH1) R132H mutation and d-2-hydroxyglutarate stimulate glutamine metabolism under hypoxia.
Abstract
Abstract
Mutations in the cytosolic NADP(+)-dependent isocitrate dehydrogenase (IDH1) occur in several types of cancer, and altered cellular metabolism associated with IDH1 mutations presents unique therapeutic opportunities. By altering IDH1, these mutations target a critical step in reductive glutamine metabolism, the metabolic pathway that converts glutamine ultimately to acetyl-CoA for biosynthetic processes. While IDH1-mutated cells are sensitive to therapies that target glutamine metabolism, the effect of IDH1 mutations on reductive glutamine metabolism remains poorly understood. To explore this issue, we investigated the effect of a knock-in, single-codon IDH1-R132H mutation on the metabolism of the HCT116 colorectal adenocarcinoma cell line. Here we report the R132H-isobolome by using targeted (13)C isotopomer tracer fate analysis to trace the metabolic fate of glucose and glutamine in this system. We show that introduction of the R132H mutation into IDH1 up-regulates the contribution of glutamine to lipogenesis in hypoxia, but not in normoxia. Treatment of cells with a d-2-hydroxyglutarate (d-2HG) ester recapitulated these changes, indicating that the alterations observed in the knocked-in cells were mediated by d-2HG produced by the IDH1 mutant. These studies provide a dynamic mechanistic basis for metabolic alterations observed in IDH1-mutated tumors and uncover potential therapeutic targets in IDH1-mutated cancers.
Cancer cell 2011 jan
Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of $\alpha$-ketoglutarate-dependent dioxygenases.
Abstract
Abstract
IDH1 and IDH2 mutations occur frequently in gliomas and acute myeloid leukemia, leading to simultaneous loss and gain of activities in the production of $\alpha$-ketoglutarate ($\alpha$-KG) and 2-hydroxyglutarate (2-HG), respectively. Here we demonstrate that 2-HG is a competitive inhibitor of multiple $\alpha$-KG-dependent dioxygenases, including histone demethylases and the TET family of 5-methlycytosine (5mC) hydroxylases. 2-HG occupies the same space as $\alpha$-KG does in the active site of histone demethylases. Ectopic expression of tumor-derived IDH1 and IDH2 mutants inhibits histone demethylation and 5mC hydroxylation. In glioma, IDH1 mutations are associated with increased histone methylation and decreased 5-hydroxylmethylcytosine (5hmC). Hence, tumor-derived IDH1 and IDH2 mutations reduce $\alpha$-KG and accumulate an $\alpha$-KG antagonist, 2-HG, leading to genome-wide histone and DNA methylation alterations.