(-)-Blebbistatin
Non-muscle myosin II (NM II) ATPase inhibitor
概要
(-)-Blebbistatin is a selective cell-permeable inhibitor of non-muscle myosin II ATPases (Kovács et al.; Straight et al.). It rapidly and reversibly inhibits Mg-ATPase activity and in vitro motility of non-muscle myosin IIA and IIB for several species (IC₅₀ = 0.5-5.0 μM), while poorly inhibiting smooth muscle myosin (IC₅₀ = 80 μM) (Limouze et al.).
MAINTENANCE AND SELF-RENEWAL
· Increases human pluripotent stem cell (hPSC) survival and cloning efficiency after dissociation to single cells, downstream of ROCK inhibition (Chen et al.; Ohgushi et al.; Walker et al.; Xu et al.).
· Enables hPSC to be cultured on microcarriers without surface coating (Chen et al.)
· Inhibits differentiation of human mesenchymal stem cells (McBeath et al.; Engler et al.).
MAINTENANCE AND SELF-RENEWAL
· Increases human pluripotent stem cell (hPSC) survival and cloning efficiency after dissociation to single cells, downstream of ROCK inhibition (Chen et al.; Ohgushi et al.; Walker et al.; Xu et al.).
· Enables hPSC to be cultured on microcarriers without surface coating (Chen et al.)
· Inhibits differentiation of human mesenchymal stem cells (McBeath et al.; Engler et al.).
Alternative Names
Not applicable
Cell Type
Mesenchymal Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Maintenance
Area of Interest
Stem Cell Biology
CAS Number
856925-71-8
Chemical Formula
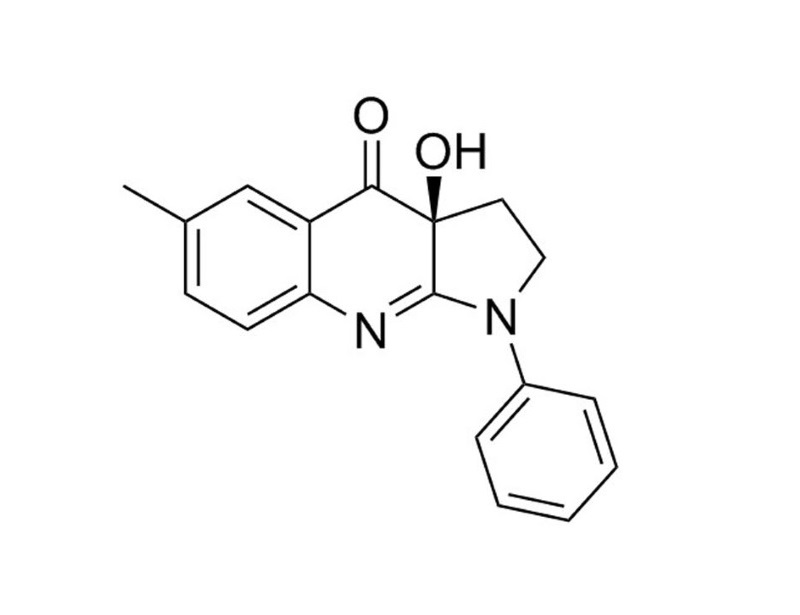
C₁₈H₁₆N₂O₂
Molecular Weight
292.3 g/mol
Purity
≥ 98%
Target
NM II ATPase
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | (-)-Blebbistatin | 72402, 72404 | All | English |
| Safety Data Sheet | (-)-Blebbistatin | 72402, 72404 | All | English |
数据及文献
Publications (10)
Proceedings of the National Academy of Sciences of the United States of America 2010 MAY
Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules.
Abstract
Abstract
Using a high-throughput chemical screen, we identified two small molecules that enhance the survival of human embryonic stem cells (hESCs). By characterizing their mechanisms of action, we discovered an essential role of E-cadherin signaling for ESC survival. Specifically, we showed that the primary cause of hESC death following enzymatic dissociation comes from an irreparable disruption of E-cadherin signaling, which then leads to a fatal perturbation of integrin signaling. Furthermore, we found that stability of E-cadherin and the resulting survival of ESCs were controlled by specific growth factor signaling. Finally, we generated mESC-like hESCs by culturing them in mESC conditions. And these converted hESCs rely more on E-cadherin signaling and significantly less on integrin signaling. Our data suggest that differential usage of cell adhesion systems by ESCs to maintain self-renewal may explain their profound differences in terms of morphology, growth factor requirement, and sensitivity to enzymatic cell dissociation.
Nature communications 2010 JAN
Non-muscle myosin II regulates survival threshold of pluripotent stem cells.
Abstract
Abstract
Human pluripotent stem (hPS) cells such as human embryonic stem (hES) and induced pluripotent stem (hiPS) cells are vulnerable under single cell conditions, which hampers practical applications; yet, the mechanisms underlying this cell death remain elusive. In this paper, we demonstrate that treatment with a specific inhibitor of non-muscle myosin II (NMII), blebbistatin, enhances the survival of hPS cells under clonal density and suspension conditions, and, in combination with a synthetic matrix, supports a fully defined environment for self-renewal. Consistent with this, genetically engineered mouse embryonic stem cells lacking an isoform of NMII heavy chain (NMHCII), or hES cells expressing a short hairpin RNA to knock down NMHCII, show greater viability than controls. Moreover, NMII inhibition increases the expression of self-renewal regulators Oct3/4 and Nanog, suggesting a mechanistic connection between NMII and self-renewal. These results underscore the importance of the molecular motor, NMII, as a novel target for chemically engineering the survival and self-renewal of hPS cells.
Cell stem cell 2010 AUG
Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells.
Abstract
Abstract
Human embryonic stem cells (hESCs), unlike mouse ones (mESCs), are vulnerable to apoptosis upon dissociation. Here, we show that the apoptosis, which is of a nonanoikis type, is caused by ROCK-dependent hyperactivation of actomyosin and efficiently suppressed by the myosin inhibitor Blebbistatin. The actomyosin hyperactivation is triggered by the loss of E-cadherin-dependent intercellular contact and also observed in dissociated mouse epiblast-derived pluripotent cells but not in mESCs. We reveal that Abr, a unique Rho-GEF family factor containing a functional Rac-GAP domain, is an indispensable upstream regulator of the apoptosis and ROCK/myosin hyperactivation. Rho activation coupled with Rac inhibition is induced in hESCs upon dissociation, but not in Abr-depleted hESCs or mESCs. Furthermore, artificial Rho or ROCK activation with Rac inhibition restores the vulnerability of Abr-depleted hESCs to dissociation-induced apoptosis. Thus, the Abr-dependent Rho-high/Rac-low" state plays a decisive role in initiating the dissociation-induced actomyosin hyperactivation and apoptosis in hESCs."
Cell stem cell 2010 AUG
Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells.
Abstract
Abstract
Human ESCs are the pluripotent precursor of the three embryonic germ layers. Human ESCs exhibit basal-apical polarity, junctional complexes, integrin-dependent matrix adhesion, and E-cadherin-dependent cell-cell adhesion, all characteristics shared by the epiblast epithelium of the intact mammalian embryo. After disruption of epithelial structures, programmed cell death is commonly observed. If individualized human ESCs are prevented from reattaching and forming colonies, their viability is significantly reduced. Here, we show that actin-myosin contraction is a critical effector of the cell death response to human ESC dissociation. Inhibition of myosin heavy chain ATPase, downregulation of myosin heavy chain, and downregulation of myosin light chain all increase survival and cloning efficiency of individualized human ESCs. ROCK inhibition decreases phosphorylation of myosin light chain, suggesting that inhibition of actin-myosin contraction is also the mechanism through which ROCK inhibitors increase cloning efficiency of human ESCs.
Cell 2006 AUG
Matrix elasticity directs stem cell lineage specification.
Abstract
Abstract
Microenvironments appear important in stem cell lineage specification but can be difficult to adequately characterize or control with soft tissues. Naive mesenchymal stem cells (MSCs) are shown here to specify lineage and commit to phenotypes with extreme sensitivity to tissue-level elasticity. Soft matrices that mimic brain are neurogenic, stiffer matrices that mimic muscle are myogenic, and comparatively rigid matrices that mimic collagenous bone prove osteogenic. During the initial week in culture, reprogramming of these lineages is possible with addition of soluble induction factors, but after several weeks in culture, the cells commit to the lineage specified by matrix elasticity, consistent with the elasticity-insensitive commitment of differentiated cell types. Inhibition of nonmuscle myosin II blocks all elasticity-directed lineage specification-without strongly perturbing many other aspects of cell function and shape. The results have significant implications for understanding physical effects of the in vivo microenvironment and also for therapeutic uses of stem cells.
Biochemical and biophysical research communications 2004 JUL
Phototoxicity and photoinactivation of blebbistatin in UV and visible light.
Abstract
Abstract
Blebbistatin was recently identified as a selective, cell-permeant inhibitor of myosin II. Because blebbistatin is likely to be used extensively with fluorescence imaging in studies of cytoskeletal dynamics, its compatibility with common excitation wavelengths was examined. Illumination of blebbistatin-treated bovine aortic endothelial cells at 365 and 450-490 nm, but not 510-560 or 590-650 nm, caused dose-dependent cell death. Illumination of blebbistatin alone at 365 and 450-490 nm changed its absorption and emission spectra, but the resultant compounds were not toxic. In addition, photoreacted blebbistatin no longer disrupted myosin distribution in cells, indicating loss of pharmacological activity. Fluorescence microscopy showed that upon illumination, blebbistatin became bound to cells and to protein-coated glass, suggesting that toxicity may arise from light-induced reaction of blebbistatin with cell proteins. Blebbistatin should be used only with careful consideration of these photochemical effects.