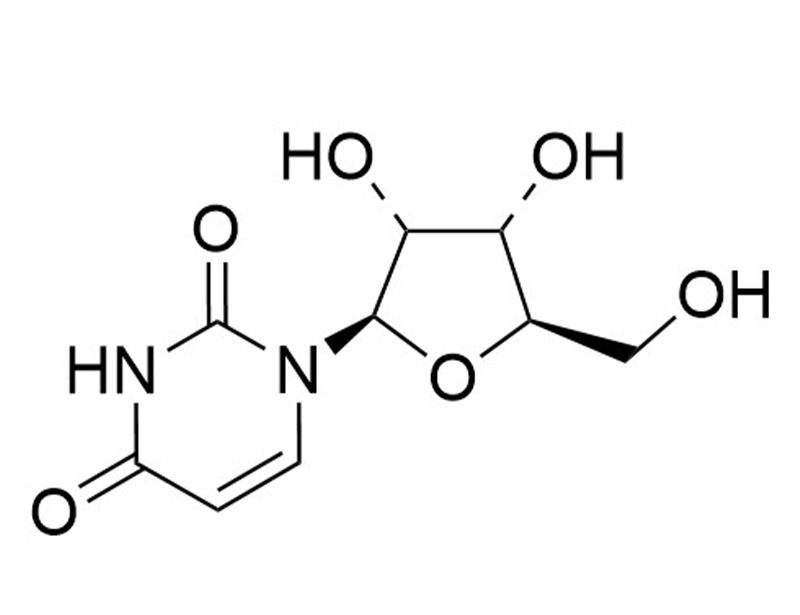
Uridine
Nucleoside for RNA synthesis
概要
Uridine is one of the five nucleosides used for RNA synthesis and as a building block for other biomolecules (Tsukimoto). Uridine 5’-diphosphate and uridine 5’-triphosphate are signaling molecules that activate the purinergic receptors (Tsukimoto). Uridine is also used as a medium supplement for culturing primary hippocampal neuron cells (François-Moutal et al.).
CANCER RESEARCH
· Uridine has been used to reduce 5-fluorouracil toxicity, without affecting its antitumor activity (Pizzorno et al.).
CANCER RESEARCH
· Uridine has been used to reduce 5-fluorouracil toxicity, without affecting its antitumor activity (Pizzorno et al.).
Alternative Names
NSC 20256; 1-β-D-Ribofuranosyluracil; Uracil-1-β-D-ribofuranoside; β-Uridine
Cell Type
Airway Cells
Area of Interest
Cancer Research, Epithelial Cell Biology
CAS Number
58-96-8
Chemical Formula
C9H12N2O6
Molecular Weight
244.2 g/mol
Purity
≥ 98%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Uridine | 100-0540, 100-0541 | All | English |
| Safety Data Sheet | Uridine | 100-0540, 100-0541 | All | English |
数据及文献
Publications (3)
Pain 2015 jul
A membrane-delimited N-myristoylated CRMP2 peptide aptamer inhibits CaV2.2 trafficking and reverses inflammatory and postoperative pain behaviors.
Abstract
Abstract
Targeting proteins within the N-type voltage-gated calcium channel (CaV2.2) complex has proven to be an effective strategy for developing novel pain therapeutics. We describe a novel peptide aptamer derived from the collapsin response mediator protein 2 (CRMP2), a CaV2.2-regulatory protein. Addition of a 14-carbon myristate group to the peptide (myr-tat-CBD3) tethered it to the membrane of primary sensory neurons near surface CaV2.2. Pull-down studies demonstrated that myr-tat-CBD3 peptide interfered with the CRMP2-CaV2.2 interaction. Quantitative confocal immunofluorescence revealed a pronounced reduction of CaV2.2 trafficking after myr-tat-CBD3 treatment and increased efficiency in disrupting CRMP2-CaV2.2 colocalization compared with peptide tat-CBD3. Consequently, myr-tat-CBD3 inhibited depolarization-induced calcium influx in sensory neurons. Voltage clamp electrophysiology experiments revealed a reduction of Ca, but not Na, currents in sensory neurons after myr-tat-CBD3 exposure. Current clamp electrophysiology experiments demonstrated a reduction in excitability of small-diameter dorsal root ganglion neurons after exposure to myr-tat-CBD3. Myr-tat-CBD3 was effective in significantly attenuating carrageenan-induced thermal hypersensitivity and reversing thermal hypersensitivity induced by a surgical incision of the plantar surface of the rat hind paw, a model of postoperative pain. These effects are compared with those of tat-CBD3-the nonmyristoylated tat-conjugated CRMP2 peptide as well as scrambled versions of CBD3 and CBD3-lacking control peptides. Our results demonstrate that the myristoyl tag enhances intracellular delivery and local concentration of the CRMP2 peptide aptamer near membrane-delimited calcium channels resulting in pronounced interference with the calcium channel complex, superior suppression of calcium influx, and better antinociceptive potential.
Biological {\&} pharmaceutical bulletin 2015
Purinergic Signaling Is a Novel Mechanism of the Cellular Response to Ionizing Radiation.
Abstract
Abstract
Recent studies suggest the effect of radiation is observed not only in irradiated cells but also in adjacent non-irradiated cells (bystander effect), although the mechanism has not yet been fully revealed. This bystander effect may be caused by intercellular communication via a gap junction or by messengers released from irradiated cells, such as reactive oxygen species, nitric oxide, or cytokines. However, an unknown mechanism is also possible in the bystander effect. On the other hand, it is known that extracellular ATP, ADP, uridine 5'-triphosphate (UTP), and uridine 5'-diphosphate (UDP), which are released from cells, act as intercellular signaling molecules by activating purinergic P2X and P2Y receptors (purinergic signaling). Recently, I have suggested these extracellular nucleotides may be novel mediators of a radiation-induced bystander effect, because our recent studies indicated that purinergic signaling is involved in important cellular responses to radiation. Our data indicate that ionizing irradiation causes activation of the transient receptor potential melastatin type 2 (TRPM2) channel, and then ATP is released from cells through the anion channel or connexin43 hemichannel mediated by the activation of a P2X7 receptor. The released nucleotides activate P2Y6 and P2Y12 receptors, which are involved in the DNA damage response after irradiation. Activation of the P2Y6 receptor is also involved in radiation-induced activation of the epithelial growth factor receptor-extracellular signal regulated protein kinase (EGFR-ERK)1/2 pathway and subsequent nuclear translocation of EGFR, which plays a role in DNA repair. Further, the induction of an antioxidant after irradiation is also mediated by the activation of the P2Y receptor. In conclusion, purinergic signaling could play an important role in the protective cellular response to ionizing irradiation.
Biochimica et biophysica acta 2002 jul
Homeostatic control of uridine and the role of uridine phosphorylase: a biological and clinical update.
Abstract
Abstract
Uridine, a pyrimidine nucleoside essential for the synthesis of RNA and bio-membranes, is a crucial element in the regulation of normal physiological processes as well as pathological states. The biological effects of uridine have been associated with the regulation of the cardio-circulatory system, at the reproduction level, with both peripheral and central nervous system modulation and with the functionality of the respiratory system. Furthermore, uridine plays a role at the clinical level in modulating the cytotoxic effects of fluoropyrimidines in both normal and neoplastic tissues. The concentration of uridine in plasma and tissues is tightly regulated by cellular transport mechanisms and by the activity of uridine phosphorylase (UPase), responsible for the reversible phosphorolysis of uridine to uracil. We have recently completed several studies designed to define the mechanisms regulating UPase expression and better characterize the multiple biological effects of uridine. Immunohistochemical analysis and co-purification studies have revealed the association of UPase with the cytoskeleton and the cellular membrane. The characterization of the promoter region of UPase has indicated a direct regulation of its expression by the tumor suppressor gene p53. The evaluation of human surgical specimens has shown elevated UPase activity in tumor tissue compared to paired normal tissue.