Trichostatin A
Epigenetic modifier; Inhibits histone deacetylase (HDAC)1 and HDAC6
概要
Trichostatin A is a potent and reversible inhibitor of Histone Deacetylase (HDAC), therefore acting as an epigenetic modifier by preventing the removal of acetyl groups from lysine residues on histone tails. Trichostatin A inhibits both class I and class II HDACs, including HDAC1 (IC₅₀ = 6 nM), HDAC4 (IC₅₀ = 38 nM), and HDAC6 (IC₅₀ = 8.6 nM). (Furumai et al.; Yoshida et al.)
REPROGRAMMING
· Increases the reprogramming efficiency of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells (Huangfu et al.).
· Resets epigenetic memory in mouse iPS cells, in combination with 5-Azacytidine (Kim et al.).
·Increases the efficiency of cloned mouse embryo development by somatic cell nuclear transfer (Kishigami et al.).
MAINTENANCE AND SELF-RENEWAL
· Prevents dedifferentiation of primary rat hepatocytes in culture, maintaining liver-specific cellular functions (Henkens et al.).
DIFFERENTIATION
· Promotes differentiation of hepatocytes from human mesenchymal stem cells (Snykers et al.).
REPROGRAMMING
· Increases the reprogramming efficiency of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells (Huangfu et al.).
· Resets epigenetic memory in mouse iPS cells, in combination with 5-Azacytidine (Kim et al.).
·Increases the efficiency of cloned mouse embryo development by somatic cell nuclear transfer (Kishigami et al.).
MAINTENANCE AND SELF-RENEWAL
· Prevents dedifferentiation of primary rat hepatocytes in culture, maintaining liver-specific cellular functions (Henkens et al.).
DIFFERENTIATION
· Promotes differentiation of hepatocytes from human mesenchymal stem cells (Snykers et al.).
Alternative Names
TSA
Cell Type
Hepatic Cells, Mesenchymal Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Maintenance, Reprogramming
Area of Interest
Epithelial Cell Biology, Stem Cell Biology
CAS Number
58880-19-6
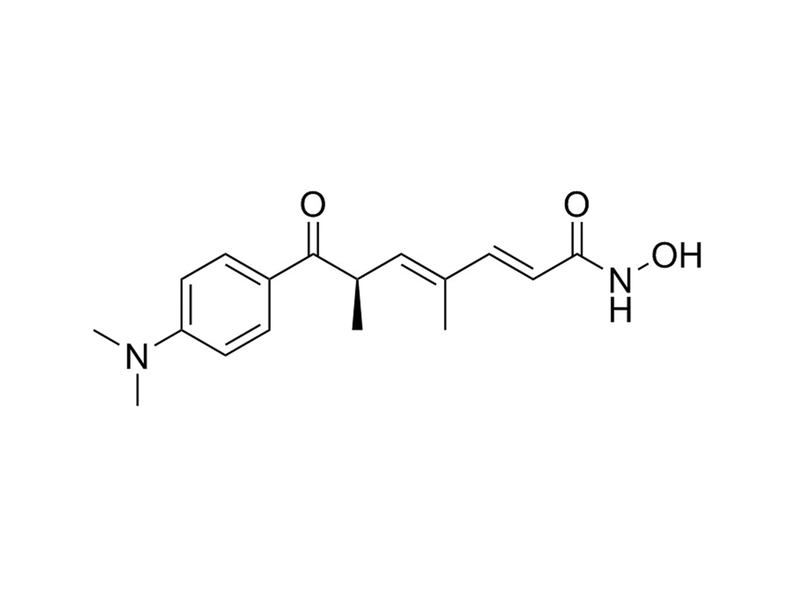
Chemical Formula
C₁₇H₂₂N₂O₃
Molecular Weight
302.4 g/mol
Purity
≥ 98%
Pathway
Epigenetic
Target
HDAC
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Trichostatin A | 72282, 72284 | All | English |
| Safety Data Sheet | Trichostatin A | 72282, 72284 | All | English |
数据及文献
Publications (7)
Nature 2010 SEP
Epigenetic memory in induced pluripotent stem cells.
Abstract
Abstract
Somatic cell nuclear transfer and transcription-factor-based reprogramming revert adult cells to an embryonic state, and yield pluripotent stem cells that can generate all tissues. Through different mechanisms and kinetics, these two reprogramming methods reset genomic methylation, an epigenetic modification of DNA that influences gene expression, leading us to hypothesize that the resulting pluripotent stem cells might have different properties. Here we observe that low-passage induced pluripotent stem cells (iPSCs) derived by factor-based reprogramming of adult murine tissues harbour residual DNA methylation signatures characteristic of their somatic tissue of origin, which favours their differentiation along lineages related to the donor cell, while restricting alternative cell fates. Such an 'epigenetic memory' of the donor tissue could be reset by differentiation and serial reprogramming, or by treatment of iPSCs with chromatin-modifying drugs. In contrast, the differentiation and methylation of nuclear-transfer-derived pluripotent stem cells were more similar to classical embryonic stem cells than were iPSCs. Our data indicate that nuclear transfer is more effective at establishing the ground state of pluripotency than factor-based reprogramming, which can leave an epigenetic memory of the tissue of origin that may influence efforts at directed differentiation for applications in disease modelling or treatment.
Nat Biotechnol 2008
Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds
Abstract
Abstract
Reprogramming of mouse and human somatic cells can be achieved by ectopic expression of transcription factors, but with low efficiencies. We report that DNA methyltransferase and histone deacetylase (HDAC) inhibitors improve reprogramming efficiency. In particular, valproic acid (VPA), an HDAC inhibitor, improves reprogramming efficiency by more than 100-fold, using Oct4-GFP as a reporter. VPA also enables efficient induction of pluripotent stem cells without introduction of the oncogene c-Myc.
Toxicology and applied pharmacology 2007 JAN
Trichostatin A, a critical factor in maintaining the functional differentiation of primary cultured rat hepatocytes.
Abstract
Abstract
Histone deacetylase inhibitors (HDI) have been shown to increase differentiation-related gene expression in several tumor-derived cell lines by hyperacetylating core histones. Effects of HDI on primary cultured cells, however, have hardly been investigated. In the present study, the ability of trichostatin A (TSA), a prototype hydroxamate HDI, to counteract the loss of liver-specific functions in primary rat hepatocyte cultures has been investigated. Upon exposure to TSA, it was found that the cell viability of the cultured hepatocytes and their albumin secretion as a function of culture time were increased. TSA-treated hepatocytes also better maintained cytochrome P450 (CYP)-mediated phase I biotransformation capacity, whereas the activity of phase II glutathione S-transferases (GST) was not affected. Western blot and qRT-PCR analysis of CYP1A1, CYP2B1 and CYP3A11 protein and mRNA levels, respectively, further revealed that TSA acts at the transcriptional level. In addition, protein expression levels of the liver-enriched transcription factors (LETFs) hepatic nuclear factor 4 alpha (HNF4alpha) and CCAAT/enhancer binding protein alpha (C/EBPalpha) were accordingly increased by TSA throughout culture time. In conclusion, these findings indicate that TSA plays a major role in the preservation of the differentiated hepatic phenotype in culture. It is suggested that the effects of TSA on CYP gene expression are mediated via controlling the expression of LETFs.
BMC developmental biology 2007 JAN
Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow.
Abstract
Abstract
BACKGROUND The capability of human mesenchymal stem cells (hMSC) derived of adult bone marrow to undergo in vitro hepatic differentiation was investigated. RESULTS Exposure of hMSC to a cocktail of hepatogenic factors [(fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF), insulin-transferrin-sodium-selenite (ITS) and dexamethasone)] failed to induce hepatic differentiation. Sequential exposure to these factors (FGF-4, followed by HGF, followed by HGF+ITS+dexamethasone), however, resembling the order of secretion during liver embryogenesis, induced both glycogen-storage and cytokeratin (CK)18 expression. Additional exposure of the cells to trichostatin A (TSA) considerably improved endodermal differentiation, as evidenced by acquisition of an epithelial morphology, chronological expression of hepatic proteins, including hepatocyte-nuclear factor (HNF)-3beta, alpha-fetoprotein (AFP), CK18, albumin (ALB), HNF1alpha, multidrug resistance-associated protein (MRP)2 and CCAAT-enhancer binding protein (C/EBP)alpha, and functional maturation, i.e. upregulated ALB secretion, urea production and inducible cytochrome P450 (CYP)-dependent activity. CONCLUSION hMSC are able to undergo mesenchymal-to-epithelial transition. TSA is hereby essential to promote differentiation of hMSC towards functional hepatocyte-like cells.
Biochemical and biophysical research communications 2006 FEB
Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer.
Abstract
Abstract
The low success rate of animal cloning by somatic cell nuclear transfer (SCNT) is believed to be associated with epigenetic errors including abnormal DNA hypermethylation. Recently, we elucidated by using round spermatids that, after nuclear transfer, treatment of zygotes with trichostatin A (TSA), an inhibitor of histone deacetylase, can remarkably reduce abnormal DNA hypermethylation depending on the origins of transferred nuclei and their genomic regions [S. Kishigami, N. Van Thuan, T. Hikichi, H. Ohta, S. Wakayama. E. Mizutani, T. Wakayama, Epigenetic abnormalities of the mouse paternal zygotic genome associated with microinsemination of round spermatids, Dev. Biol. (2005) in press]. Here, we found that 5-50 nM TSA-treatment for 10 h following oocyte activation resulted in more efficient in vitro development of somatic cloned embryos to the blastocyst stage from 2- to 5-fold depending on the donor cells including tail tip cells, spleen cells, neural stem cells, and cumulus cells. This TSA-treatment also led to more than 5-fold increase in success rate of mouse cloning from cumulus cells without obvious abnormality but failed to improve ES cloning success. Further, we succeeded in establishment of nuclear transfer-embryonic stem (NT-ES) cells from TSA-treated cloned blastocyst at a rate three times higher than those from untreated cloned blastocysts. Thus, our data indicate that TSA-treatment after SCNT in mice can dramatically improve the practical application of current cloning techniques.
Proceedings of the National Academy of Sciences of the United States of America 2001 JAN
Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin.
Abstract
Abstract
Trichostatin A (TSA) and trapoxin (TPX) are potent inhibitors of histone deacetylases (HDACs). TSA is proposed to block the catalytic reaction by chelating a zinc ion in the active-site pocket through its hydroxamic acid group. On the other hand, the epoxyketone is suggested to be the functional group of TPX capable of alkylating the enzyme. We synthesized a novel TPX analogue containing a hydroxamic acid instead of the epoxyketone. The hybrid compound cyclic hydroxamic acid-containing peptide (CHAP) 1 inhibited HDAC1 at low nanomolar concentrations. The HDAC1 inhibition by CHAP1 was reversible as it was by TSA, in contrast to the irreversible inhibition by TPX. CHAP with an aliphatic chain length of five, which corresponded to that of acetylated lysine, was stronger than those with other lengths. These results suggest that TPX is a substrate mimic and that the replacement of the epoxyketone with the hydroxamic acid converted TPX to an inhibitor chelating the zinc like TSA. Interestingly, HDAC6, but not HDAC1 or HDAC4, was resistant to TPX and CHAP1, whereas TSA inhibited these HDACs to a similar extent. HDAC6 inhibition by TPX at a high concentration was reversible, probably because HDAC6 is not alkylated by TPX. We further synthesized the counterparts of all known naturally occurring cyclic tetrapeptides containing the epoxyketone. HDAC1 was highly sensitive to all these CHAPs much more than HDAC6, indicating that the structure of the cyclic tetrapeptide framework affects the target enzyme specificity. These results suggest that CHAP is a unique lead to develop isoform-specific HDAC inhibitors.