Thiazovivin
RHO/ROCK pathway inhibitor; Inhibits ROCK
概要
Thiazovivin is a selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK), a serine/threonine kinase that plays a role in cell polarity, contraction, and actin cytoskeleton reorganization (Xu et al.). Thiazovivin is effective at 5-fold-lower concentrations than another common ROCK inhibitor Y-27632 (Catalog #72302; Xu et al.).
MAINTENANCE AND SELF-RENEWAL
· Promotes survival of human embryonic stem (ES) cells during dissociation by stabilizing E-cadherin and improves cell attachment (Xu et al.).
· Promotes survival of single human induced pluripotent stem (iPS) cells during transfection for TALEN-mediated genome editing (Sun and Zhao).
REPROGRAMMING
· Increases the efficiency of reprogramming human somatic cells to iPS cells, in combination with PD0325091 and SB431542 (Lin et al.).
· Increases the efficiency of reprogramming human cord blood mononuclear cells to iPS cells (Hu et al.).
MAINTENANCE AND SELF-RENEWAL
· Promotes survival of human embryonic stem (ES) cells during dissociation by stabilizing E-cadherin and improves cell attachment (Xu et al.).
· Promotes survival of single human induced pluripotent stem (iPS) cells during transfection for TALEN-mediated genome editing (Sun and Zhao).
REPROGRAMMING
· Increases the efficiency of reprogramming human somatic cells to iPS cells, in combination with PD0325091 and SB431542 (Lin et al.).
· Increases the efficiency of reprogramming human cord blood mononuclear cells to iPS cells (Hu et al.).
Alternative Names
Tzv
Cell Type
Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Maintenance, Reprogramming
Area of Interest
Cell Line Development, Stem Cell Biology
CAS Number
1226056-71-8
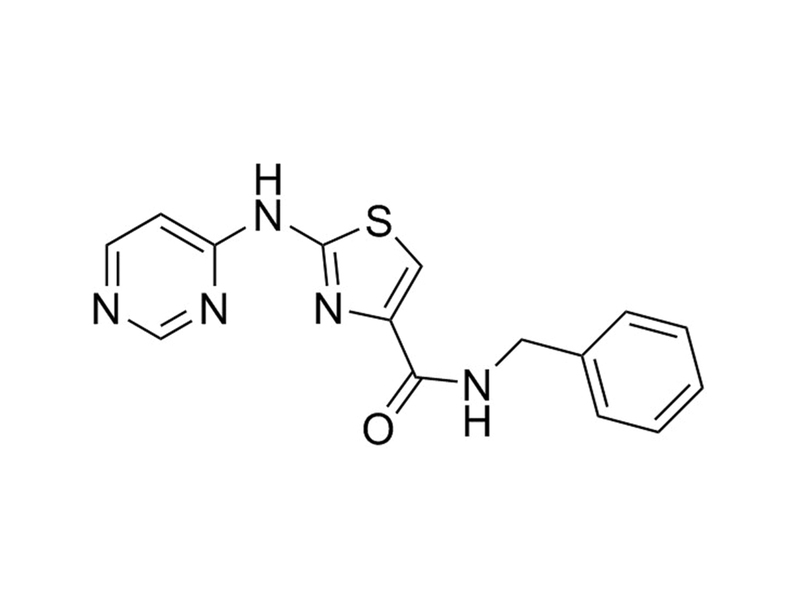
Chemical Formula
C₁₅H₁₃N₅OS
Molecular Weight
311.4 g/mol
Purity
≥ 98%
Pathway
RHO/ROCK
Target
ROCK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Thiazovivin | 72252, 72254, 100-0247 | All | English |
| Safety Data Sheet | Thiazovivin | 72252, 72254 | All | English |
| Safety Data Sheet | Thiazovivin | 100-0247 | All | English |
数据及文献
Publications (4)
Biotechnology and Bioengineering 2014 MAY
Seamless correction of the sickle cell disease mutation of the HBB gene in human induced pluripotent stem cells using TALENs.
Abstract
Abstract
Sickle cell disease (SCD) is the most common human genetic disease which is caused by a single mutation of human β-globin (HBB) gene. The lack of long-term treatment makes the development of reliable cell and gene therapies highly desirable. Disease-specific patient-derived human induced pluripotent stem cells (hiPSCs) have great potential for developing novel cell and gene therapies. With the disease-causing mutations corrected in situ, patient-derived hiPSCs can restore normal cell functions and serve as a renewable autologous cell source for the treatment of genetic disorders. Here we successfully utilized transcription activator-like effector nucleases (TALENs), a recently emerged novel genome editing tool, to correct the SCD mutation in patient-derived hiPSCs. The TALENs we have engineered are highly specific and generate minimal off-target effects. In combination with piggyBac transposon, TALEN-mediated gene targeting leaves no residual ectopic sequences at the site of correction and the corrected hiPSCs retain full pluripotency and a normal karyotype. Our study demonstrates an important first step of using TALENs for the treatment of genetic diseases such as SCD, which represents a significant advance toward hiPSC-based cell and gene therapies.
Blood 2011 APR
Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells.
Abstract
Abstract
Reprogramming blood cells to induced pluripotent stem cells (iPSCs) provides a novel tool for modeling blood diseases in vitro. However, the well-known limitations of current reprogramming technologies include low efficiency, slow kinetics, and transgene integration and residual expression. In the present study, we have demonstrated that iPSCs free of transgene and vector sequences could be generated from human BM and CB mononuclear cells using non-integrating episomal vectors. The reprogramming described here is up to 100 times more efficient, occurs 1-3 weeks faster compared with the reprogramming of fibroblasts, and does not require isolation of progenitors or multiple rounds of transfection. Blood-derived iPSC lines lacked rearrangements of IGH and TCR, indicating that their origin is non-B- or non-T-lymphoid cells. When cocultured on OP9, blood-derived iPSCs could be differentiated back to the blood cells, albeit with lower efficiency compared to fibroblast-derived iPSCs. We also generated transgene-free iPSCs from the BM of a patient with chronic myeloid leukemia (CML). CML iPSCs showed a unique complex chromosomal translocation identified in marrow sample while displaying typical embryonic stem cell phenotype and pluripotent differentiation potential. This approach provides an opportunity to explore banked normal and diseased CB and BM samples without the limitations associated with virus-based methods.
Proceedings of the National Academy of Sciences of the United States of America 2010 MAY
Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules.
Abstract
Abstract
Using a high-throughput chemical screen, we identified two small molecules that enhance the survival of human embryonic stem cells (hESCs). By characterizing their mechanisms of action, we discovered an essential role of E-cadherin signaling for ESC survival. Specifically, we showed that the primary cause of hESC death following enzymatic dissociation comes from an irreparable disruption of E-cadherin signaling, which then leads to a fatal perturbation of integrin signaling. Furthermore, we found that stability of E-cadherin and the resulting survival of ESCs were controlled by specific growth factor signaling. Finally, we generated mESC-like hESCs by culturing them in mESC conditions. And these converted hESCs rely more on E-cadherin signaling and significantly less on integrin signaling. Our data suggest that differential usage of cell adhesion systems by ESCs to maintain self-renewal may explain their profound differences in terms of morphology, growth factor requirement, and sensitivity to enzymatic cell dissociation.
Nature methods 2009 NOV
A chemical platform for improved induction of human iPSCs.
Abstract
Abstract
The slow kinetics and low efficiency of reprogramming methods to generate human induced pluripotent stem cells (iPSCs) impose major limitations on their utility in biomedical applications. Here we describe a chemical approach that dramatically improves (200-fold) the efficiency of iPSC generation from human fibroblasts, within seven days of treatment. This will provide a basis for developing safer, more efficient, nonviral methods for reprogramming human somatic cells.