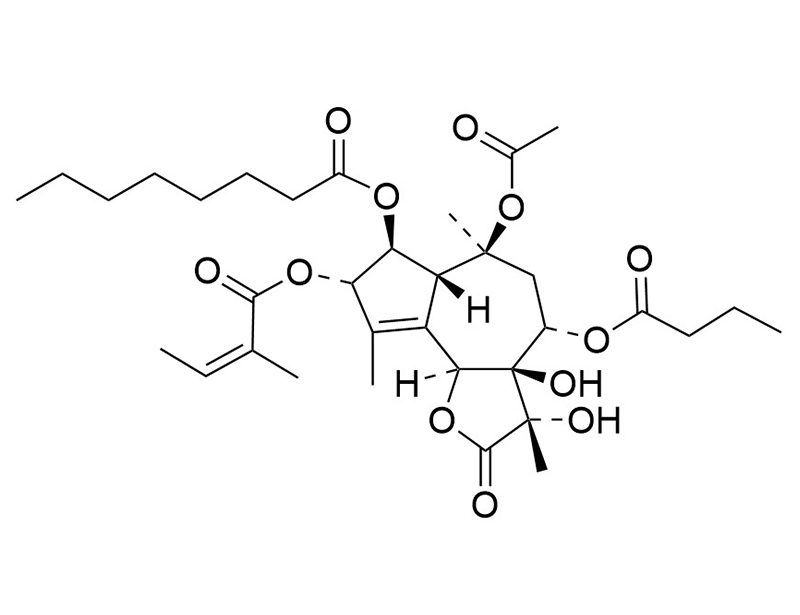
Thapsigargin
Inhibits sarco-endoplasmic reticulum Ca2+-ATPases (SERCAs)
概要
Thapsigargin is a sesquiterpene lactone and a cell-permeable inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases (SERCAs; Sabała et al.; Wictome et al.). It also induces autophagy in mammalian cells (Ding et al.).
CANCER RESEARCH
· Induces apoptosis in thymocytes in rats (Jiang et al.).
· Induces endoplasmic reticulum stress and autophagy in mammalian cells (Ding et al.).
CANCER RESEARCH
· Induces apoptosis in thymocytes in rats (Jiang et al.).
· Induces endoplasmic reticulum stress and autophagy in mammalian cells (Ding et al.).
Cell Type
Cancer Cells and Cell Lines
Area of Interest
Autophagy, Cancer Research
CAS Number
67526-95-8
Chemical Formula
C34H50O12
Molecular Weight
650.8 g/mol
Purity
≥ 97%
Pathway
JNK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Thapsigargin | 100-0568, 100-0569 | All | English |
| Safety Data Sheet | Thapsigargin | 100-0568, 100-0569 | All | English |
数据及文献
Publications (4)
The Journal of biological chemistry 2007 feb
Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival.
Abstract
Abstract
Autophagy is a cellular response to adverse environment and stress, but its significance in cell survival is not always clear. Here we show that autophagy could be induced in the mammalian cells by chemicals, such as A23187, tunicamycin, thapsigargin, and brefeldin A, that cause endoplasmic reticulum stress. Endoplasmic reticulum stress-induced autophagy is important for clearing polyubiquitinated protein aggregates and for reducing cellular vacuolization in HCT116 colon cancer cells and DU145 prostate cancer cells, thus mitigating endoplasmic reticulum stress and protecting against cell death. In contrast, autophagy induced by the same chemicals does not confer protection in a normal human colon cell line and in the non-transformed murine embryonic fibroblasts but rather contributes to cell death. Thus the impact of autophagy on cell survival during endoplasmic reticulum stress is likely contingent on the status of cells, which could be explored for tumor-specific therapy.
The Biochemical journal 1995 sep
Binding of sesquiterpene lactone inhibitors to the Ca(2+)-ATPase.
Abstract
Abstract
The mechanism of inhibition of the Ca(2+)-ATPase from sarcoplasmic reticulum by the sesquiterpene lactones thapsigargin, trilobolide and thapsivillosin A (TvA) has been determined. A decrease in the affinity of the ATPase for Ca2+ is observed in the presence of the inhibitors (I), consistent with a shift in the E1/E2 equilibrium for the ATPase towards E2 forms. Amounts of inhibitor beyond a 1:1 molar ratio with ATPase produce no further decrease in affinity for Ca2+, inconsistent with the formation of a dead-end complex. Measurements of the rate of quenching of the tryptophan fluorescence of the ATPase by TvA are consistent with an association step to give E2I followed by an isomerization to a modified state E2AI. The kinetics of the reversal of the effects of TvA by Ca2+ at sub-stoichiometric amounts of TvA are bi-exponential, with a fast component whose rate is independent of TvA concentration and equal to the rate observed in the absence of TvA, and a slow component whose rate decreases with increasing TvA concentration. These observations are also consistent with the formation of a modified state E2AI following the initial binding of I to E2. The equilibrium constant E2AI/E2I increases in the order TvA {\textless} trilobolide {\textless} thapsigargin. The results suggest that the effects of the inhibitors on the overall ratio of E2 to E1 forms of the ATPase follow largely from the formation of E2AI from E2I, and that binding constants are very similar for E1Ca2, E1 and E2.
Experimental cell research 1994 may
Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca(2+)-ATPase inhibitor thapsigargin.
Abstract
Abstract
The endoplasmic reticular Ca(2+)-ATPase inhibitor, thapsigargin, was used to study the role of an increase in cytosolic free calcium concentration ([Ca2+]i) as a signal for the activation of thymocyte apoptosis. Treatment of rat thymocytes with thapsigargin resulted in an early sustained increase in [Ca2+]i followed by extensive DNA fragmentation. Agarose gel electrophoresis revealed that the pattern of DNA fragments was typical of endonuclease-mediated internucleosomal cleavage. In addition, confocal microscopy studies showed the formation of apoptotic nuclei in thapsigargin-treated thymocytes. The concentrations of thapsigargin required to induce DNA fragmentation and [Ca2+]i increase in thymocytes were identical and so were the kinetics of thapsigargin-induced DNA fragmentation and formation of apoptotic nuclei. The lowest concentration of thapsigargin needed to activate apoptosis was 1 nM. Thapsigargin-induced [Ca2+]i increase and thymocyte apoptosis were inhibited in cells incubated in nominally Ca(2+)-free medium or pretreated with the intracellular Ca2+ chelator, bis-(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid/acetoxymethyl ester. Removal of extracellular free Ca2+ with 5 mM EGTA at different time points after thapsigargin addition revealed a time dependency of about 2 h for the sustained increase in [Ca2+]i to trigger apoptosis in thymocytes. Thus, we conclude that the signal provided by the thapsigargin-induced [Ca2+]i increase is sufficient to activate thymocyte apoptosis.
Acta biochimica Polonica 1993
Thapsigargin: potent inhibitor of Ca2+ transport ATP-ases of endoplasmic and sarcoplasmic reticulum.
Abstract