TTNPB
Retinoid pathway activator; Activates retinoic acid receptor (RAR)
概要
TTNPB is an analog of retinoic acid that potently and selectively activates retinoic acid receptors (RAR; EC₅₀ = 21, 4, and 2.4 nM for RARα, RARβ, and RARγ, respectively; Beard et al.; Wong et al.).
DIFFERENTIATION
· In combination with CHIR99021 or Activin A, induces intermediate mesoderm formation from human or mouse pluripotent stem cells, respectively (Araoka et al.; Oeda et al.).
· Promotes neuronal differentiation in cultured chick caudal neural plate explants (Diez del Corral et al.).
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Tranylcypromine, Valproic Acid, 3-Deazaneplanocin A, and E-616452 (Hou et al.).
CANCER RESEARCH
· Induces the in vitro growth and differentiation to granulocytes of myeloid progenitors isolated from myelodysplastic syndrome (MDS) patients (Fabian et al.).
DIFFERENTIATION
· In combination with CHIR99021 or Activin A, induces intermediate mesoderm formation from human or mouse pluripotent stem cells, respectively (Araoka et al.; Oeda et al.).
· Promotes neuronal differentiation in cultured chick caudal neural plate explants (Diez del Corral et al.).
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Tranylcypromine, Valproic Acid, 3-Deazaneplanocin A, and E-616452 (Hou et al.).
CANCER RESEARCH
· Induces the in vitro growth and differentiation to granulocytes of myeloid progenitors isolated from myelodysplastic syndrome (MDS) patients (Fabian et al.).
Alternative Names
AGN 191183; Arotinoid Acid; Ro 13-7410
Cell Type
Cancer Cells and Cell Lines, Mesoderm, PSC-Derived, Neural Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Reprogramming
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
71441-28-6
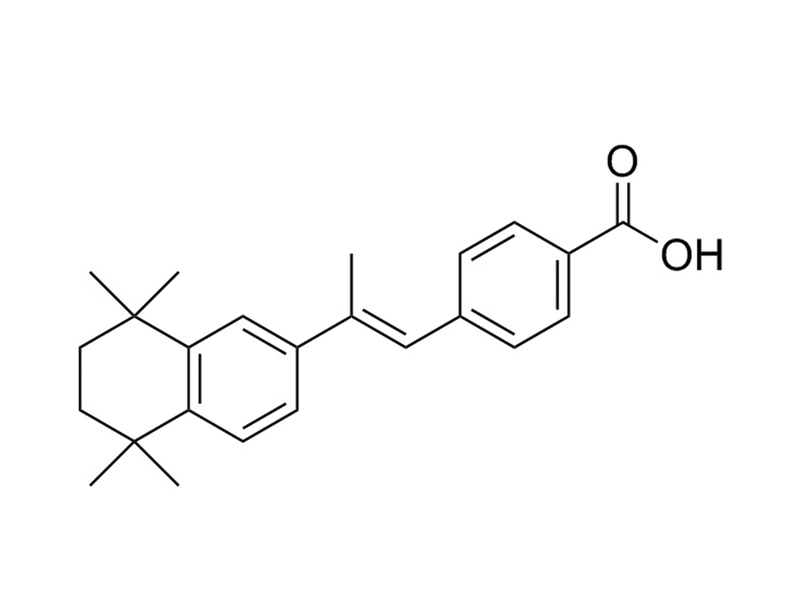
Chemical Formula
C₂₄H₂₈O₂
Molecular Weight
348.5 g/mol
Purity
≥ 98%
Pathway
Retinoid
Target
RAR
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | TTNPB | 72892 | All | English |
| Safety Data Sheet | TTNPB | 72892 | All | English |
数据及文献
Publications (10)
PloS one 2014 JAN
Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods.
Abstract
Abstract
The first step in developing regenerative medicine approaches to treat renal diseases using pluripotent stem cells must be the generation of intermediate mesoderm (IM), an embryonic germ layer that gives rise to kidneys. In order to achieve this goal, establishing an efficient, stable and low-cost method for differentiating IM cells using small molecules is required. In this study, we identified two retinoids, AM580 and TTNPB, as potent IM inducers by high-throughput chemical screening, and established rapid (five days) and efficient (80% induction rate) IM differentiation from human iPSCs using only two small molecules: a Wnt pathway activator, CHIR99021, combined with either AM580 or TTNPB. The resulting human IM cells showed the ability to differentiate into multiple cell types that constitute adult kidneys, and to form renal tubule-like structures. These small molecule differentiation methods can bypass the mesendoderm step, directly inducing IM cells by activating Wnt, retinoic acid (RA), and bone morphogenetic protein (BMP) pathways. Such methods are powerful tools for studying kidney development and may potentially provide cell sources to generate renal lineage cells for regenerative therapy.
The International journal of developmental biology 2013 JAN
Induction of intermediate mesoderm by retinoic acid receptor signaling from differentiating mouse embryonic stem cells.
Abstract
Abstract
Renal lineages including kidney are derived from intermediate mesoderm, which are differentiated from a subset of caudal undifferentiated mesoderm. The inductive mechanisms of mammalian intermediate mesoderm and renal lineages are still poorly understood. Mouse embryonic stem cells (mESCs) can be a good in vitro model to reconstitute the developmental pathway of renal lineages and to analyze the mechanisms of the sequential differentiation. We examined the effects of Activin A and retinoic acid (RA) on the induction of intermediate mesoderm from mESCs under defined, serum-free, adherent, monolayer culture conditions. We measured the expression level of intermediate mesodermal marker genes and examined the developmental potential of the differentiated cells into kidney using an ex vivo transplantation assay. Adding Activin A followed by RA to mESC cultures induced the expression of marker genes and proteins for intermediate mesoderm, odd-skipped related 1 (Osr1) and Wilms Tumor 1 (Wt1). These differentiated cells integrated into laminin-positive tubular cells and Pax2-positive renal cells in cultured embryonic kidney explants. We demonstrated that intermediate mesodermal marker expression was also induced by RA receptor (RAR) agonist, but not by retinoid X receptor (RXR) agonists. Furthermore, the expression of these markers was decreased by RAR antagonists. We directed the differentiation of mESCs into intermediate mesoderm using Activin A and RA and revealed the role of RAR signaling in this differentiation. These methods and findings will improve our understanding of renal lineage development and could contribute to the regenerative medicine of kidney.
Science (New York, N.Y.) 2013 AUG
Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds.
Abstract
Abstract
Pluripotent stem cells can be induced from somatic cells, providing an unlimited cell resource, with potential for studying disease and use in regenerative medicine. However, genetic manipulation and technically challenging strategies such as nuclear transfer used in reprogramming limit their clinical applications. Here, we show that pluripotent stem cells can be generated from mouse somatic cells at a frequency up to 0.2% using a combination of seven small-molecule compounds. The chemically induced pluripotent stem cells resemble embryonic stem cells in terms of their gene expression profiles, epigenetic status, and potential for differentiation and germline transmission. By using small molecules, exogenous master genes" are dispensable for cell fate reprogramming. This chemical reprogramming strategy has potential use in generating functional desirable cell types for clinical applications."
Differentiation 2009 NOV
Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells.
Abstract
Abstract
Differentiation of embryonic and adult myogenic progenitors undergoes a complex series of cell rearrangements and specification events which are controlled by distinct gene regulatory networks. Delineation of the molecular mechanisms that regulate skeletal muscle specification and formation should be important for understanding congenital myopathies and muscular degenerative diseases. Retinoic acid (RA) signaling plays an important role in development. However, the role of RA signaling in adult myogenic progenitors is poorly understood. Here, we investigate the role of RA signaling in regulating myogenic differentiation of myoblastic progenitor cells. Using the mouse myoblast progenitor C2C12 line as a model, we have found that the endogenous expression of most RAR and RXR isotypes is readily detected. While the nuclear receptor co-repressors are highly expressed, two of the three nuclear receptor co-activators and the enzymes involved in RA synthesis are expressed at low level or undetectable, suggesting that the RA signaling pathway may be repressed in myogenic progenitors. Using the alpha-myosin heavy chain promoter-driven reporter (MyHC-GLuc), we have demonstrated that either ATRA or 9CRA is able to effectively induce myogenic differentiation, which can be synergistically enhanced when both ATRA and 9CRA are used. Upon ATRA and 9CRA treatment of C2C12 cells the expression of late myogenic markers significantly increases. We have further shown that adenovirus-mediated exogenous expression of RARalpha and/or RXRalpha is able to effectively induce myogenic differentiation in a ligand-independent fashion. Morphologically, ATRA- and 9CRA-treated C2C12 cells exhibit elongated cell body and become multi-nucleated myoblasts, and even form myoblast fusion. Ultrastructural analysis under transmission electron microscope reveals that RA-treated myogenic progenitor cells exhibit an abundant presence of muscle fibers. Therefore, our results strongly suggest that RA signaling may play an important role in regulating myogenic differentiation.
Neuron 2003 SEP
Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension.
Abstract
Abstract
Vertebrate body axis extension involves progressive generation and subsequent differentiation of new cells derived from a caudal stem zone; however, molecular mechanisms that preserve caudal progenitors and coordinate differentiation are poorly understood. FGF maintains caudal progenitors and its attenuation is required for neuronal and mesodermal differentiation and to position segment boundaries. Furthermore, somitic mesoderm promotes neuronal differentiation in part by downregulating Fgf8. Here we identify retinoic acid (RA) as this somitic signal and show that retinoid and FGF pathways have opposing actions. FGF is a general repressor of differentiation, including ventral neural patterning, while RA attenuates Fgf8 in neuroepithelium and paraxial mesoderm, where it controls somite boundary position. RA is further required for neuronal differentiation and expression of key ventral neural patterning genes. Our data demonstrate that FGF and RA pathways are mutually inhibitory and suggest that their opposing actions provide a global mechanism that controls differentiation during axis extension.
Journal of medicinal chemistry 2000 AUG
Identification of a chemical tool for the orphan nuclear receptor FXR.
Abstract