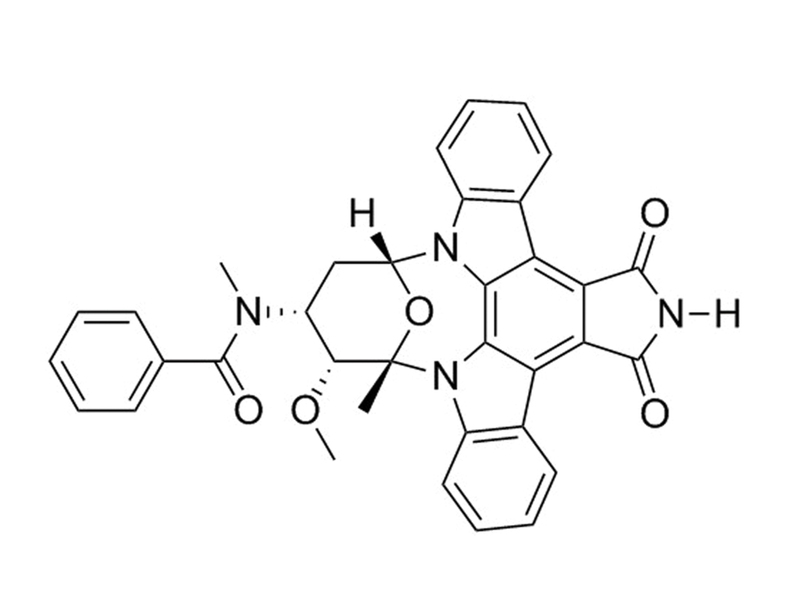
Stauprimide
Inhibitor of NME2 localization; Suppresses c-MYC expression
概要
Stauprimide specifically inhibits the nuclear localization of NME2, which results in the suppression of c-MYC—a key regulator of pluripotency—thereby priming cells for differentiation (Zhu et al.).
DIFFERENTIATION
· Enhances cytokine-mediated directed differentiation of mouse and human pluripotent stem cells to multiple lineages, including definitive endoderm, neural progenitors, and mesodermal derivatives such as cardiomyocytes (Zhu et al.; Tahamtani et al.).
DIFFERENTIATION
· Enhances cytokine-mediated directed differentiation of mouse and human pluripotent stem cells to multiple lineages, including definitive endoderm, neural progenitors, and mesodermal derivatives such as cardiomyocytes (Zhu et al.; Tahamtani et al.).
Alternative Names
N-Benzoyl-7-oxo Staurosporine
Cell Type
Endoderm, PSC-Derived, Mesoderm, PSC-Derived, Neural Cells, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
154589-96-5
Chemical Formula
C₃₅H₂₈N₄O₅
Molecular Weight
584.6 g/mol
Purity
≥ 98%
Target
NME2
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | Stauprimide | 72652 | All | English |
| Product Information Sheet 2 | Stauprimide | 72652 | All | English |
| Safety Data Sheet | Stauprimide | 72652 | All | English |
数据及文献
Publications (2)
Cell journal 2014 FEB
Stauprimide Priming of Human Embryonic Stem Cells toward Definitive Endoderm.
Abstract
Abstract
OBJECTIVE: In vitro production of a definitive endoderm (DE) is an important issue in stem cell-related differentiation studies and it can assist with the production of more efficient endoderm derivatives for therapeutic applications. Despite tremendous progress in DE differentiation of human embryonic stem cells (hESCs), researchers have yet to discover universal, efficient and cost-effective protocols. MATERIALS AND METHODS: In this experimental study, we have treated hESCs with 200 nM of Stauprimide (Spd) for one day followed by activin A (50 ng/ml; A50) for the next three days (Spd-A50). In the positive control group, hESCs were treated with Wnt3a (25 ng/ml) and activin A (100 ng/ml) for the first day followed by activin A for the next three days (100 ng/ml; W/A100-A100). RESULTS: Gene expression analysis showed up regulation of DE-specific marker genes (SOX17, FOXA2 and CXCR4) comparable to that observed in the positive control group. Expression of the other lineage specific markers did not significantly change (ptextless0.05). We also obtained the same gene expression results using another hESC line. The use of higher concentrations of Spd (400 and 800 nM) in the Spd-A50 protocol caused an increase in the expression SOX17 as well as a dramatic increase in mortality rate of the hESCs. A lower concentration of activin A (25 ng/ml) was not able to up regulate the DE-specific marker genes. Then, A50 was replaced by inducers of definitive endoderm; IDE1/2 (IDE1 and IDE2), two previously reported small molecule (SM) inducers of DE, in our protocol (Spd-IDE1/2). This replacement resulted in the up regulation of visceral endoderm (VE) marker (SOX7) but not DE-specific markers. Therefore, while the Spd-A50 protocol led to DE production, we have shown that IDE1/2 could not fully replace activin A in DE induction of hESCs. CONCLUSION: These findings can assist with the design of more efficient chemically-defined protocols for DE induction of hESCs and lead to a better understanding of the different signaling networks that are involved in DE differentiation of hESCs.
Cell stem cell 2009 MAY
A small molecule primes embryonic stem cells for differentiation.
Abstract
Abstract
Embryonic stem cells (ESCs) are an attractive source of cells for disease modeling in vitro and may eventually provide access to cells/tissues for the treatment of many degenerative diseases. However, applications of ESC-derived cell types are largely hindered by the lack of highly efficient methods for lineage-specific differentiation. Using a high-content screen, we have identified a small molecule, named stauprimide, that increases the efficiency of the directed differentiation of mouse and human ESCs in synergy with defined extracellular signaling cues. Affinity-based methods revealed that stauprimide interacts with NME2 and inhibits its nuclear localization. This, in turn, leads to downregulation of c-Myc, a key regulator of the pluripotent state. Thus, our findings identify a chemical tool that primes ESCs for efficient differentiation through a mechanism that affects c-Myc expression, and this study points to an important role for NME2 in ESC self-renewal.