Reversine
Adenosine receptor, non-muscle myosin II (NM II), MEK1, and aurora kinase inhibitor
概要
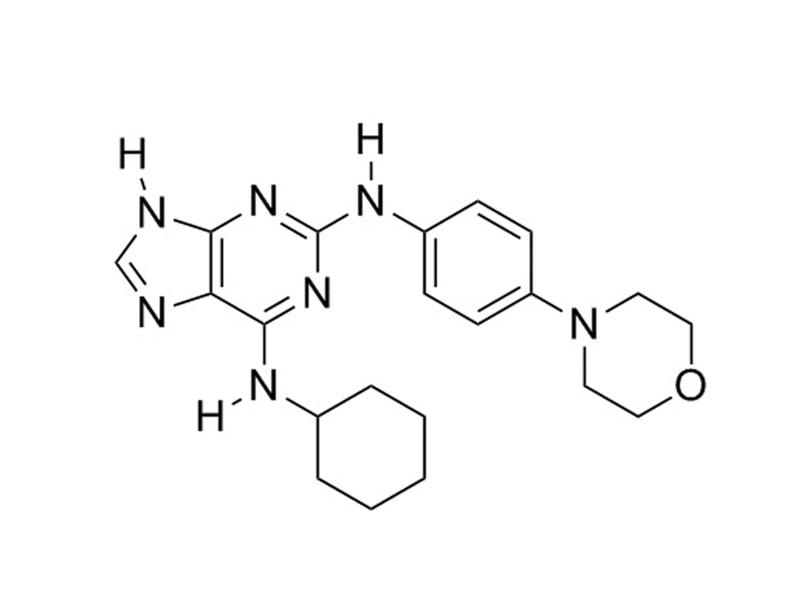
Reversine is a 2,6-disubstituted purine derivative that inhibits the human A3 adenosine receptor, nonmuscle myosin II heavy chain, mitogen activated extra-cellular signal regulated kinase-1 (MEK1), and Aurora B kinase (Chen et al.; Perreira et al.; D’Alise et al.).
REPROGRAMMING
· Induces dedifferentiation of lineage-committed mouse myoblasts to multipotent progenitors with osteogenic and adipogenic potential (Chen et al., 2007; Chen et al., 2004).
· Induces dedifferentiation of primary mouse and human dermal fibroblasts to myogenic-competent cells (Anastasia et al.).
· Induces dedifferentiation of annulus fibrosus cells to multipotent mesenchymal progenitors that have the potential to develop along chondrogenic, osteogenic, or adipogenic lineages (Saraiya et al.).
CANCER RESEARCH
· Inhibits cell proliferation and induces polyploidy in the PC-3, HeLa, CWR22Rv1, and DU-145 cancer cell lines (Hsieh et al.).
· Induces differentiation in the human embryonal carcinoma cell line NT2, and in the human promyelocytic leukemia cell line HL60 (D’Alise et al.).
· Blocks proliferation, causes failure of cytokinesis, and induces polyploidy in multiple cancer cell lines (D’Alise et al.).
REPROGRAMMING
· Induces dedifferentiation of lineage-committed mouse myoblasts to multipotent progenitors with osteogenic and adipogenic potential (Chen et al., 2007; Chen et al., 2004).
· Induces dedifferentiation of primary mouse and human dermal fibroblasts to myogenic-competent cells (Anastasia et al.).
· Induces dedifferentiation of annulus fibrosus cells to multipotent mesenchymal progenitors that have the potential to develop along chondrogenic, osteogenic, or adipogenic lineages (Saraiya et al.).
CANCER RESEARCH
· Inhibits cell proliferation and induces polyploidy in the PC-3, HeLa, CWR22Rv1, and DU-145 cancer cell lines (Hsieh et al.).
· Induces differentiation in the human embryonal carcinoma cell line NT2, and in the human promyelocytic leukemia cell line HL60 (D’Alise et al.).
· Blocks proliferation, causes failure of cytokinesis, and induces polyploidy in multiple cancer cell lines (D’Alise et al.).
Alternative Names
Not applicable
Cell Type
Cancer Cells and Cell Lines, Leukemia/Lymphoma Cells, Mesenchymal Stem and Progenitor Cells, Myogenic Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Reprogramming
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
656820-32-5
Chemical Formula
C₂₁H₂₇N₇O
Molecular Weight
393.5 g/mol
Purity
> 98%
Pathway
Adenosine, Aurora Kinase, MEK/ERK
Target
Adenosine Receptor, Aurora Kinase B, MEK1, NM II
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Reversine | 72612, 72614 | All | English |
| Safety Data Sheet | Reversine | 72612, 72614 | All | English |
数据及文献
Publications (7)
Tissue engineering. Part A 2010 APR
Reversine enhances generation of progenitor-like cells by dedifferentiation of annulus fibrosus cells.
Abstract
Abstract
The aim of this study was to determine if treatment with reversine, a purine analog, promoted generation of skeletal progenitor cells from lineage-committed annulus fibrosus cells. Reversine modulated cell growth, morphology, and the actin cytoskeleton of annulus fibrosus cells. Microarray profiling coupled with Ingenuity Pathway Analysis revealed that reversine treatment resulted in a significant expression change in many genes including those required for cell-cell interaction, cell movement, cell growth, and development. Further analysis revealed that there was involvement of gene networks concerned with cellular assembly and organization, DNA replication and repair, tissue morphology, and cell-to-cell signaling. The gene expression profile was dependent on reversine concentration. In osteogenic media, cells pretreated with 300 nM reversine exhibited an increased induction in alkaline phosphatase activity and enhanced expression of alkaline phosphatase, bone sialoprotein, osteocalcin, and collagen type I mRNA. Maintained in adipogenic media, the reversine-pretreated annulus cells displayed evidence of adipogenic differentiation: accumulation of cytosolic lipid droplets and increased expression of PPAR-gamma2, LPL, and Fabp mRNA. In chondrogenic media, cells pretreated with reversine exhibited marked increase in the induction of aggrecan, collagen types II, IX, and XI, and versican. It is concluded that reversine treatment induced annulus fibrosus cell plasticity and promoted their differentiation along mesenchymal lineages. This agent could be used to generate skeletal progenitor cells to orchestrate the repair of the intervertebral disc.
Molecular cancer therapeutics 2008 MAY
Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells.
Abstract
Abstract
The demonstration that the small synthetic molecule reversine [2-(4-morpholinoanilino)-N6-cyclohexyladenine] promotes the dedifferentiation of committed cells into multipotent progenitor-type cells has raised hopes on the exploitation of this small chemical tool for the generation of stem cells. Here, we show that reversine causes a failure in cytokinesis and induces polyploidization. These effects of reversine are due to the inhibition of Aurora A and B, two related kinases that are implicated in several aspects of mitosis and that are frequently amplified and overexpressed in human tumors. Reversine inhibits the phosphorylation of histone H3, a direct downstream target of Aurora kinases. Similarly to the Aurora kinase inhibitor VX-680, which has recently entered phase II clinical trials for cancer treatment, reversine inhibited colony formation of leukemic cells from patients with acute myeloid leukemia but was significantly less toxic than VX-680 on cells from healthy donors. The crystal structure of the reversine-Aurora B kinase complex shows that reversine is a novel class of ATP-competitive Aurora kinase inhibitors. Thus, although our studies raise serious doubts on the application of reversine in regenerative medicine, they support the paradigm that reversine might be a useful agent in cancer chemotherapy.
Proceedings of the National Academy of Sciences of the United States of America 2007 JUN
Reversine increases the plasticity of lineage-committed mammalian cells.
Abstract
Abstract
Previously, a small molecule, reversine, was identified that reverses lineage-committed murine myoblasts to a more primitive multipotent state. Here, we show that reversine can increase the plasticity of C2C12 myoblasts at the single-cell level and that reversine-treated cells gain the ability to differentiate into osteoblasts and adipocytes under lineage-specific inducing conditions. Moreover, reversine is active in multiple cell types, including 3T3E1 osteoblasts and human primary skeletal myoblasts. Biochemical and cellular experiments suggest that reversine functions as a dual inhibitor of nonmuscle myosin II heavy chain and MEK1, and that both activities are required for reversine's effect. Inhibition of MEK1 and nonmuscle myosin II heavy chain results in altered cell cycle and changes in histone acetylation status, but other factors also may contribute to the activity of reversine, including activation of the PI3K signaling pathway.
International journal of oncology 2007 DEC
The 2,6-disubstituted purine reversine induces growth arrest and polyploidy in human cancer cells.
Abstract
Abstract
Reversine (RV) is the synthetic purine identified from a protein kinase-based screen of purine mimetics and it has been shown to induce muscle myoblast differentiation into progenitor cells that can be further converted into other cell lineages. Since protein kinases play a pivotal role in cell cycle control, we hypothesize that RV might affect the proliferation of cancer cells. Herein we report that RV inhibited growth of cultured human tumor cells, respectively, PC-3, HeLa, CWR22Rv1, and DU-145 cells, and induced accumulation of polyploidal cells with textgreater or =4N DNA content. However, RV was without effect on growth of normal prostate epithelial cells. RV-treated PC-3 cells showed enlarged nuclei and an estimated 100-fold increase in cell size. Moreover, PC-3 cells treated with RV for 2-4 days were accompanied by a marked increase in the expression of p21(WAF1), a modest elevation in the levels of cyclin D3 and CDK6 and concomitantly, also a substantial reduction in cyclin B and CDK1. These results suggest that RV may induce polyploidy and increase in cell size by up-regulating p21(WAF1) and cyclin D3/CDK6, while simultaneously suppressing the expression of cyclin B and CDK1.
Cell death and differentiation 2006 DEC
Reversine-treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle.
Abstract
Abstract
Stem cells hold a great potential for the regeneration of damaged tissues in cardiovascular or musculoskeletal diseases. Unfortunately, problems such as limited availability, control of cell fate, and allograft rejection need to be addressed before therapeutic applications may become feasible. Generation of multipotent progenitors from adult differentiated cells could be a very attractive alternative to the limited in vitro self-renewal of several types of stem cells. In this direction, a recently synthesized unnatural purine, named reversine, has been proposed to induce reversion of adult cells to a multipotent state, which could be then converted into other cell types under appropriate stimuli. Our study suggests that reversine treatment transforms primary murine and human dermal fibroblasts into myogenic-competent cells both in vitro and in vivo. Moreover, this is the first study to demonstrate that plasticity changes arise in primary mouse and human cells following reversine exposure.
Journal of medicinal chemistry 2005 JUL
Reversine" and its 2-substituted adenine derivatives as potent and selective A3 adenosine receptor antagonists."
Abstract
Abstract
The dedifferentiation agent reversine" [2-(4-morpholinoanilino)-N(6)-cyclohexyladenine 2] was found to be a moderately potent antagonist for the human A(3) adenosine receptor (AR) with a K(i) value of 0.66 microM. This result prompted an exploration of the structure-activity relationship of related derivatives�