Refametinib
MEK/ERK pathway inhibitor; Inhibits MEK1 and MEK2
概要
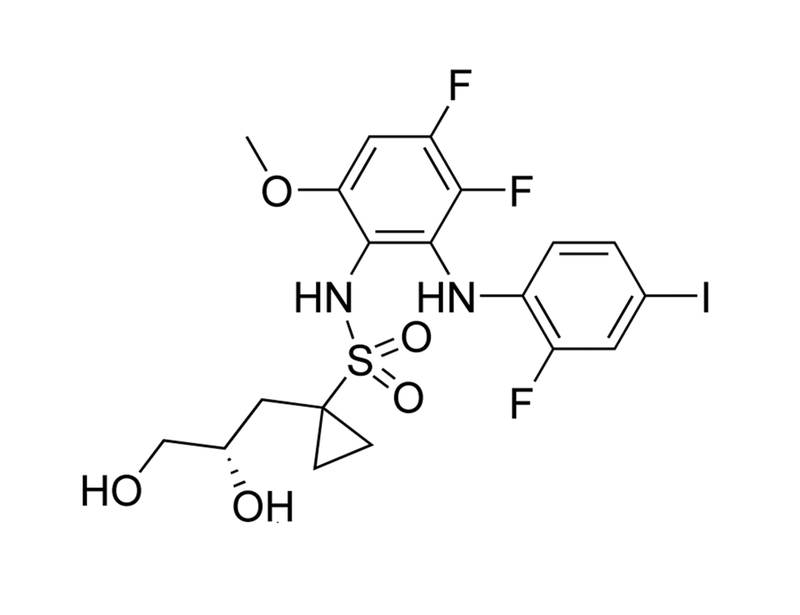
Refametinib is an inhibitor of both mitogen-activated protein kinase kinases 1 (MEK1) and 2 (MEK2) with IC₅₀ values of 19 and 47 nM respectively. It binds in an allosteric site adjacent to the ATP pocket and is selective for MEK1/2 versus 205 other kinases (Iverson et al.).
CANCER RESEARCH
· Inhibits growth of cancer cell lines in vitro, including those expressing B-RAF mutation V600E (Iverson et al.).
· Inhibits tumor growth in various xenograft models including human melanoma A375 and human colon cancer Colo205 cell lines, and primary pancreatic cancers (Iverson et al.; Chang et al.).
· Synergistically induces apoptosis in pancreatic cancer cell lines when combined with Erlotinib, an epidermal growth factor receptor (EGFR) inhibitor (Diep et al.).
· Synergistically inhibits tumor growth in hepatocellular carcinoma rodent models when combined with Sorafenib, an inhibitor of the tyrosine kinases vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR; Schmieder et al.).
CANCER RESEARCH
· Inhibits growth of cancer cell lines in vitro, including those expressing B-RAF mutation V600E (Iverson et al.).
· Inhibits tumor growth in various xenograft models including human melanoma A375 and human colon cancer Colo205 cell lines, and primary pancreatic cancers (Iverson et al.; Chang et al.).
· Synergistically induces apoptosis in pancreatic cancer cell lines when combined with Erlotinib, an epidermal growth factor receptor (EGFR) inhibitor (Diep et al.).
· Synergistically inhibits tumor growth in hepatocellular carcinoma rodent models when combined with Sorafenib, an inhibitor of the tyrosine kinases vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR; Schmieder et al.).
Alternative Names
BAY-86-9766; RDEA119
Cell Type
Cancer Cells and Cell Lines
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research
CAS Number
923032-37-5
Chemical Formula
C₁₉H₂₀F₃IN₂O₅S
Molecular Weight
572.3 g/mol
Purity
≥ 95%
Pathway
MEK/ERK
Target
MEK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Refametinib | 73372, 73374 | All | English |
| Safety Data Sheet | Refametinib | 73372, 73374 | All | English |
数据及文献
Publications (4)
Neoplasia 2013
Allosteric MEK1/2 Inhibitor Refametinib (BAY 86-9766) in Combination with Sorafenib Exhibits Antitumor Activity in Preclinical Murine and Rat Models of Hepatocellular Carcinoma
Abstract
Abstract
OBJECTIVE: The objectives of the study were to evaluate the allosteric mitogen-activated protein kinase kinase (MEK) inhibitor BAY 86-9766 in monotherapy and in combination with sorafenib in orthotopic and subcutaneous hepatocellular carcinoma (HCC) models with different underlying etiologies in two species. DESIGN: Antiproliferative potential of BAY 86-9766 and synergistic effects with sorafenib were studied in several HCC cell lines. Relevant pathway signaling was studied in MH3924a cells. For in vivo testing, the HCC cells were implanted subcutaneously or orthotopically. Survival and mode of action (MoA) were analyzed. RESULTS: BAY 86-9766 exhibited potent antiproliferative activity in HCC cell lines with half-maximal inhibitory concentration values ranging from 33 to 762 nM. BAY 86-9766 was strongly synergistic with sorafenib in suppressing tumor cell proliferation and inhibiting phosphorylation of the extracellular signal-regulated kinase (ERK). BAY 86-9766 prolonged survival in Hep3B xenografts, murine Hepa129 allografts, and MH3924A rat allografts. Additionally, tumor growth, ascites formation, and serum alpha-fetoprotein levels were reduced. Synergistic effects in combination with sorafenib were shown in Huh-7, Hep3B xenografts, and MH3924A allografts. On the signaling pathway level, the combination of BAY 86-9766 and sorafenib led to inhibition of the upregulatory feedback loop toward MEK phosphorylation observed after BAY 86-9766 monotreatment. With regard to the underlying MoA, inhibition of ERK phosphorylation, tumor cell proliferation, and microvessel density was observed in vivo. CONCLUSION: BAY 86-9766 shows potent single-agent antitumor activity and acts synergistically in combination with sorafenib in preclinical HCC models. These results support the ongoing clinical development of BAY 86-9766 and sorafenib in advanced HCC.
Clinical cancer research : an official journal of the American Association for Cancer Research 2011
Synergistic effect between erlotinib and MEK inhibitors in KRAS wild-type human pancreatic cancer cells.
Abstract
Abstract
PURPOSE: The combination of erlotinib and gemcitabine has shown a small but statistically significant survival advantage when compared with gemcitabine alone in patients with advanced pancreatic cancer. However, the overall survival rate with the erlotinib and gemcitabine combination is still low. In this study, we sought to identify gene targets that, when inhibited, would enhance the activity of epidermal growth factor receptor (EGFR)-targeted therapies in pancreatic cancer cells. EXPERIMENTAL DESIGN: A high-throughput RNA interference (RNAi) screen was carried out to identify candidate genes. Selected gene hits were further confirmed and mechanisms of action were further investigated using various assays. RESULTS: Six gene hits from siRNA screening were confirmed to significantly sensitize BxPC-3 pancreatic cancer cells to erlotinib. One of the hits, mitogen-activated protein kinase (MAPK) 1, was selected for further mechanistic studies. Combination treatments of erlotinib and two MAP kinase kinase (MEK) inhibitors, RDEA119 and AZD6244, showed significant synergistic effect for both combinations (RDEA119-erlotinib and AZD6244-erlotinib) compared with the corresponding single drug treatments in pancreatic cancer cell lines with wild-type KRAS (BxPC-3 and Hs 700T) but not in cell lines with mutant KRAS (MIA PaCa-2 and PANC-1). The enhanced antitumor activity of the combination treatment was further verified in the BxPC-3 and MIA PaCa-2 mouse xenograft model. Examination of the MAPK signaling pathway by Western blotting indicated effective inhibition of the EGFR signaling by the drug combination in KRAS wild-type cells but not in KRAS mutant cells. CONCLUSIONS: Overall, our results suggest that combination therapy of an EGFR and MEK inhibitors may have enhanced efficacy in patients with pancreatic cancer.
BMC cancer 2010
Antitumour activity of a potent MEK inhibitor RDEA119/BAY 869766 combined with rapamycin in human orthotopic primary pancreatic cancer xenografts.
Abstract
Abstract
BACKGROUND: Combining MEK inhibitors with other signalling pathway inhibitors or conventional cytotoxic drugs represents a promising new strategy against cancer. RDEA119/BAY 869766 is a highly potent and selective MEK1/2 inhibitor undergoing phase I human clinical trials. The effects of RDEA119/BAY 869766 as a single agent and in combination with rapamycin were studied in 3 early passage primary pancreatic cancer xenografts, OCIP19, 21, and 23, grown orthotopically. METHODS: Anti-cancer effects were determined in separate groups following chronic drug exposure. Effects on cell cycle and downstream signalling were examined by flow cytometry and western blot, respectively. Plasma RDEA119 concentrations were measured to monitor the drug accumulation in vivo. RESULTS: RDEA119/BAY 869766 alone or in combination with rapamycin showed significant growth inhibition in all the 3 models, with a significant decrease in the percentage of cells in S-phase, accompanied by a large decrease in bromodeoxyuridine labelling and cell cycle arrest predominantly in G1. The S6 ribosomal protein was inhibited to a greater extent with combination treatment in all the three models. Blood plasma pharmacokinetic analyses indicated that RDEA119 levels achieved in vivo are similar to those that produce target inhibition and cell cycle arrest in vitro. CONCLUSIONS: Agents targeting the ERK and mTOR pathway have anticancer activity in primary xenografts, and these results support testing this combination in pancreatic cancer patients.
Cancer research 2009
RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer.
Abstract
Abstract
The RAS-RAF-mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway provides numerous opportunities for targeted oncology therapeutics. In particular, the MEK enzyme is attractive due to high selectivity for its target ERK and the central role that activated ERK plays in driving cell proliferation. The structural, pharmacologic, and pharmacokinetic properties of RDEA119/BAY 869766, an allosteric MEK inhibitor, are presented. RDEA119/BAY 869766 is selectively bound directly to an allosteric pocket in the MEK1/2 enzymes. This compound is highly efficacious at inhibiting cell proliferation in several tumor cell lines in vitro. In vivo, RDEA119/BAY 869766 exhibits potent activity in xenograft models of melanoma, colon, and epidermal carcinoma. RDEA119/BAY 869766 exhibits complete suppression of ERK phosphorylation at fully efficacious doses in mice. RDEA119/BAY 869766 shows a tissue selectivity that reduces its potential for central nervous system-related side effects. Using pharmacokinetic and pharmacodynamic data, we show that maintaining adequate MEK inhibition throughout the dosing interval is likely more important than achieving high peak levels because greater efficacy was achieved with more frequent but lower dosing. Based on its longer half-life in humans than in mice, RDEA119/BAY 869766 has the potential for use as a once- or twice-daily oral treatment for cancer. RDEA119/BAY 869766, an exquisitely selective, orally available MEK inhibitor, has been selected for clinical development because of its potency and favorable pharmacokinetic profile.