Raloxifene
Selective estrogen receptor modulator
概要
Raloxifene is a selective estrogen receptor modulator (SERM) that exhibits agonistic (estrogenic) activity in bone cells without stimulating ER activity in breast or uterine tissues (Black et al.).
DIFFERENTIATION
· Enhances osteoblast differentiation in mouse bone marrow and human osteoblast cultures (Qu et al.; Viereck et al.).
· Inhibits osteoclast differentiation in primary human bone marrow mononuclear cell cultures (Ramalho et al.).
· Reduced endodermal differentiation (HNF-4α expression) in embryoid bodies derived from human embryonic stem (ES) cells (Kim et al.).
DIFFERENTIATION
· Enhances osteoblast differentiation in mouse bone marrow and human osteoblast cultures (Qu et al.; Viereck et al.).
· Inhibits osteoclast differentiation in primary human bone marrow mononuclear cell cultures (Ramalho et al.).
· Reduced endodermal differentiation (HNF-4α expression) in embryoid bodies derived from human embryonic stem (ES) cells (Kim et al.).
Alternative Names
Keoxifene; LY156758
Cell Type
Mesenchymal Stem and Progenitor Cells, Osteoblasts
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
82640-04-8
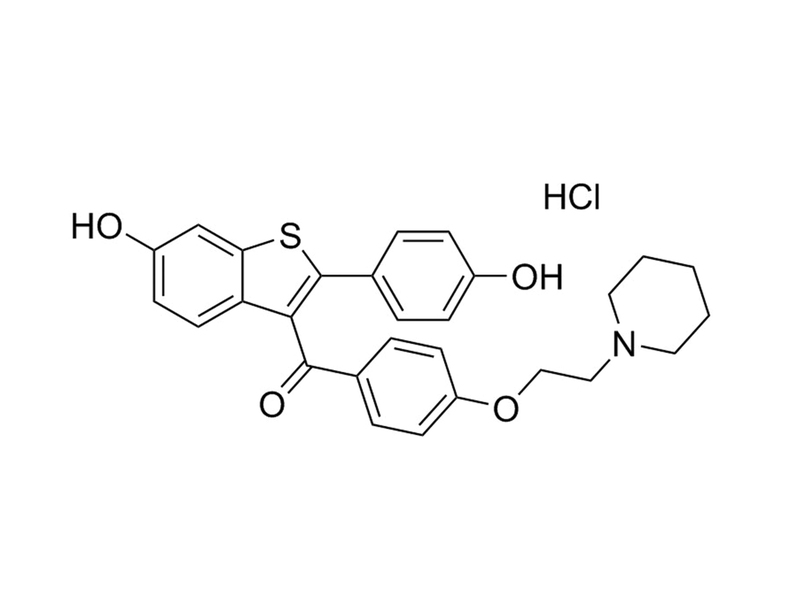
Chemical Formula
C₂₈H₂₇NO₄S · HCl
Molecular Weight
510.2 g/mol
Purity
≥ 98%
Pathway
Estrogen
Target
ER
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Raloxifene (Hydrochloride) | 72852, 72854 | All | English |
| Safety Data Sheet | Raloxifene (Hydrochloride) | 72852, 72854 | All | English |
数据及文献
Publications (5)
Reproductive sciences (Thousand Oaks, Calif.) 2013 JUN
The effect of estrogen compounds on human embryoid bodies.
Abstract
Abstract
Human embryonic stem cells are derived from the inner cell mass of preimplantation embryo at the blastocyst stage and their differentiation occurs through an intermediate step involving the formation of embryoid bodies (EBs), which are aggregates of embryonic stem cells. The EBs seem to be a powerful tool for investigating the development of embryos, as they can mimic the initial stages of embryonic development. In this study, we aimed to investigate the effect of estrogen compounds on the proliferation and differentiation of short-term and long-term cultured EBs in vitro. For this study, 10-day-old (short-term cultured) and 30-day-old (long-term cultured) EBs were subjected to estradiol (E2), estriol (E3), selective estrogen receptor modulator (raloxifene [RLX]), bisphenol A, and 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole for 7 days. To confirm the effects of estrogen treatment, ICI-182780 was added to the respective EBs for additional 7 days following estrogen treatment. Quantitative reverse transcription-polymerase chain reaction was performed to analyze the relative expression of differentiation marker genes representing the 3 germ layers. The expression of 7 marker genes, which included α-fetoprotein, hepatocyte nuclear factor (HNF)-3β, HNF-4α (endoderm), brachyury, cardiac actin ([cACT]; mesoderm), nestin (ectoderm), and Oct-4 (undifferentiated), was measured. Significantly, lower expression of HNF-4α in both short-term and long-term cultured EBs was observed after treatment of estrogen compounds compared to control. The expression of HNF-3β in short-term cultured EBs has been positively affected by E2, E3, and RLX. Regarding cACT, higher expression was observed after treatment of E2 (10(-7) mol/L) and E3 (10(-9) mol/L) in short-term cultured EBs, but opposite effects were demonstrated in long-term cultured EBs. The lower expressions of HNF-4α by E2 and RLX were negated by ICI-182780 treatment, although these findings were not statistically significant in E3-treated group. These findings suggest that estrogen compounds have effects on endodermal and mesodermal differentiation of human EBs.
The Journal of clinical endocrinology and metabolism 2003 SEP
Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts.
Abstract
Abstract
Raloxifene reduces bone loss and prevents vertebral fractures in postmenopausal women. Its skeletal effects are mediated by estrogen receptors (ER) and their modulation of paracrine osteoblastic factors. Receptor activator of nuclear factor-kappa B ligand is essential for osteoclasts and enhances bone resorption, whereas osteoprotegerin (OPG) neutralizes receptor activator of nuclear factor-kappa B ligand. Here, we assessed the effects of raloxifene on OPG production in human osteoblasts (hOB). Raloxifene enhanced gene expression of ER-alpha and progesterone receptor. Moreover, raloxifene increased OPG mRNA levels and protein secretion by hOB in a dose- and time-dependent fashion by 2- to 4-fold with a maximum effect at 10(-7) M and after 72 h (P textless 0.001). Treatment with the ER antagonist ICI 182,780 abrogated the effects of raloxifene on OPG production. Moreover, raloxifene enhanced osteoblastic differentiation markers, type 1 collagen secretion, and alkaline phosphatase activity by 3- and 2-fold, respectively (P textless 0.001). In addition, raloxifene inhibited expression of the bone-resorbing cytokine IL-6 by 25-45% (P textless 0.001). In conclusion, our data suggest that raloxifene stimulates OPG production and inhibits IL-6 production by hOB. Because OPG production increases with osteoblastic maturation, enhancement of OPG production by raloxifene could be related to its stimulatory effects on osteoblastic differentiation.
European cytokine network 2002 APR
Estradiol and raloxifene decrease the formation of multinucleate cells in human bone marrow cultures.
Abstract
Abstract
Estrogen (E2) deficiency is responsible for increased bone turnover in the postmenopausal period, and it can be prevented by estrogen replacement therapy. The way estrogen acts on bone cells is not fully understood. Human bone marrow cell cultures may be a reliable model for studying the action of steroids on osteoclastogenesis in vitro. We examine the effects of estradiol and Raloxifene, a selective estrogen receptor modulator, on human primary bone marrow cells cultured for 15 days. 17beta-estradiol and Raloxifene significantly decreased the number of tartrate-resistant acid phosphatase multinucleate cells from osteoclast precursors on day 15. Estrogen receptor alpha (ER-alpha) mRNA was present in bone marrow mononuclear cells cultured for 5 days, but there was no estrogen receptor beta (ER-beta) mRNA, suggesting that this effect was mediated by ER-alpha. 15-day cultures no longer contained ER-alpha mRNA, suggesting that estrogen acts on early events of osteoclast differentiation. Finally, 10-8 M 17beta-estradiol has no effect on the release of IL-6 and IL-6-sr into the medium of marrow mononuclear cells cultured for 5 or 15 days. Osteoclast apoptosis was not affected by estradiol or Raloxifene after 15 days of culture under our conditions. In conclusion, we have shown that both estradiol and Raloxifene inhibit osteoclast differentiation in human bone marrow mononuclear cultures. The biological effect that can mimic in vivo differentiation could be mediated through ER-alpha.
Journal of cellular biochemistry 1999 JUN
Comparative effects of estrogen and antiestrogens on differentiation of osteoblasts in mouse bone marrow culture.
Abstract
Abstract
Estrogens as well as some antiestrogens have been shown to prevent bone loss in postmenopausal women. These compounds seem to inhibit bone resorption, but their anabolic effects have been less explored. In this study, bone marrow cultures were used to compare the effect of 17beta-estradiol (E2), and two triphenylethylene derivatives, tamoxifen (TAM), and FC1271a, and a benzothiophene derivative raloxifene (RAL) on differentiation of osteoblasts. All enhanced osteoblastic differentiation of 21-day cultures as indicated by increased mineralization and bone nodule formation. All, except RAL, stimulated cell proliferation during the first 6 days of the culture. However, in the presence of RAL the content of total protein was increased in 13-day cultures. SDS-PAGE and autoradiography of [14C]-proline labeled proteins revealed elevated level of the newly synthesized collagen type I. The pure antiestrogen ICI 182,780 abolished the increase of the specific activity of alkaline phosphatase by E2, TAM, and FC1271a but not the effect of RAL on protein synthesis. Our results show that E2 as well as TAM, FC1271a, and RAL stimulate bone formation in vitro but the mechanism of the anabolic action of RAL in bone clearly differs from that of E2, TAM, and FC1271a.
The Journal of clinical investigation 1994 JAN
Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats.
Abstract
Abstract
There is a medical need for an agent with the positive effects of estrogen on bone and the cardiovascular system, but without the negative effects on reproductive tissue. Raloxifene (LY139481 HCI) is a benzothiophene derivative that binds to the estrogen receptor and inhibits the effects of estrogen on the uterus. In an ovariectomized (OVX) rat model we investigated the effects of raloxifene on bone loss (induced by estrogen deficiency), serum lipids, and uterine tissue. After oral administration of raloxifene for 5 wk (0.1-10 mg/kg per d) to OVX rats, bone mineral density in the distal femur and proximal tibia was significantly greater than that observed in OVX controls (ED50 of 0.03-0.3 mg/kg). Serum cholesterol was lower in the raloxifene-treated animals, which had a minimal effective dose of 0.1 mg/kg and an approximate oral ED50 of 0.2 mg/kg. The effects of raloxifene on bone and serum cholesterol were comparable to those of a 0.1-mg/kg per d oral dose of ethynyl estradiol. Raloxifene diverged dramatically from estrogen in its lack of significant estrogenic effects on uterine tissue. Ethynyl estradiol produced a marked elevation in a number of uterine histologic parameters (e.g., epithelial cell height, stromal eosinophilia). These data suggest that raloxifene has promise as an agent with beneficial bone and cardiovascular effects in the absence of significant uterine effects.