Puromycin
Antibiotic; Protein synthesis inhibitor
概要
Puromycin is an aminonucleoside antibiotic, derived from Streptomyces alboniger, which acts as a protein synthesis inhibitor. It binds to the target ribosome site A, where it is transferred to the growing polypeptide chain causing premature chain termination (Lührmann et al.; Rodriguez-Fonseca et al.; Azzam & Algranati). A biologically inactive form is generated when puromycin is N-acetylated by puromycin-N-acetyltransferase, allowing this gene to be used as a selective resistance marker (Vara et al. 1985).
CELL LINE DEVELOPMENT
· Selects for cells expressing puromycin-N-acetyltransferase resistance gene as a research tool (de la Luna & Ortín; Iwaki et al.).
· Useful in CRISPR/Cas9 mammalian gene editing by selecting for successful Cas9-induced knock-in with puromycin resistance gene (Park et al.).
CANCER RESEARCH
· Possesses anti-tumor activity when tested against numerous cell lines (Foley & Eagle).
CELL LINE DEVELOPMENT
· Selects for cells expressing puromycin-N-acetyltransferase resistance gene as a research tool (de la Luna & Ortín; Iwaki et al.).
· Useful in CRISPR/Cas9 mammalian gene editing by selecting for successful Cas9-induced knock-in with puromycin resistance gene (Park et al.).
CANCER RESEARCH
· Possesses anti-tumor activity when tested against numerous cell lines (Foley & Eagle).
Alternative Names
CL 13900; CL 16536; NSC 3055; PDH
Cell Type
Cancer Cells and Cell Lines, CHO Cells, Hybridomas, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Antibiotic
Area of Interest
Cancer Research, Cell Line Development, Hybridoma Generation
CAS Number
58-58-2
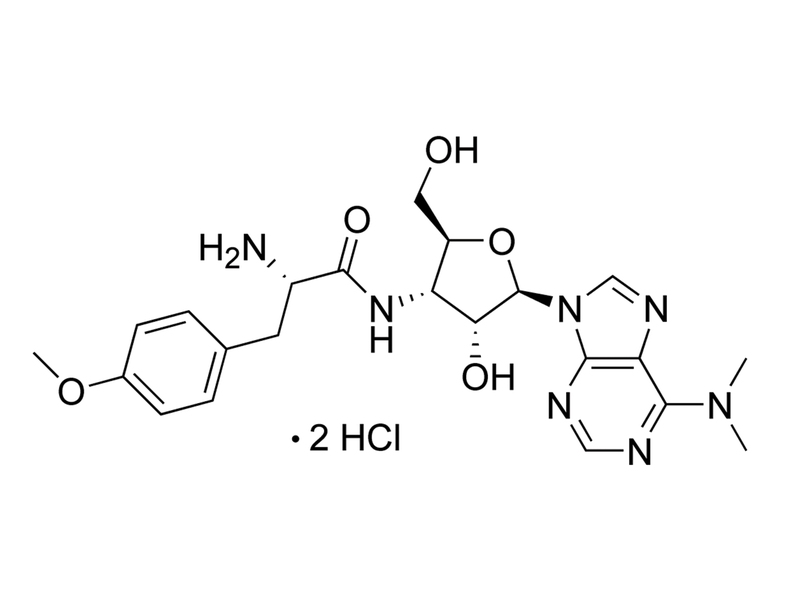
Chemical Formula
C₂₂H₂₉N₇O₅ · 2HCl
Molecular Weight
544.4 g/mol
Purity
≥ 98%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Puromycin (Dihydrochloride) | 73342, 73344 | All | English |
| Safety Data Sheet | Puromycin (Dihydrochloride) | 73342, 73344 | All | English |
数据及文献
Publications (10)
PloS one 2014
CRISPR/Cas9 allows efficient and complete knock-in of a destabilization domain-tagged essential protein in a human cell line, allowing rapid knockdown of protein function.
Abstract
Abstract
Although modulation of protein levels is an important tool for study of protein function, it is difficult or impossible to knockdown or knockout genes that are critical for cell growth or viability. For such genes, a conditional knockdown approach would be valuable. The FKBP protein-based destabilization domain (DD)-tagging approach, which confers instability to the tagged protein in the absence of the compound Shield-1, has been shown to provide rapid control of protein levels determined by Shield-1 concentration. Although a strategy to knock-in DD-tagged protein at the endogenous loci has been employed in certain parasite studies, partly due to the relative ease of knock-in as a result of their mostly haploid lifecycles, this strategy has not been demonstrated in diploid or hyperploid mammalian cells due to the relative difficulty of achieving complete knock-in in all alleles. The recent advent of CRISPR/Cas9 homing endonuclease-mediated targeted genome cleavage has been shown to allow highly efficient homologous recombination at the targeted locus. We therefore assessed the feasibility of using CRISPR/Cas9 to achieve complete knock-in to DD-tag the essential gene Treacher Collins-Franceschetti syndrome 1 (TCOF1) in human 293T cells. Using a double antibiotic selection strategy to select clones with at least two knock-in alleles, we obtained numerous complete knock-in clones within three weeks of initial transfection. DD-TCOF1 expression in the knock-in cells was Shield-1 concentration-dependent, and removal of Shield-1 resulted in destabilization of DD-TCOF1 over the course of hours. We further confirmed that the tagged TCOF1 retained the nucleolar localization of the wild-type untagged protein, and that destabilization of DD-TCOF1 resulted in impaired cell growth, as expected for a gene implicated in ribosome biogenesis. CRISPR/Cas9-mediated homologous recombination to completely knock-in a DD tag likely represents a generalizable and efficient strategy to achieve rapid modulation of protein levels in mammalian cells.
PLoS ONE 2013 NOV
Targeting of Herpes Simplex Virus 1 Thymidine Kinase Gene Sequences into the OCT4 Locus of Human Induced Pluripotent Stem Cells
Abstract
Abstract
The in vitro differentiation of human induced pluripotent stem cells (hiPSC) to generate specific types of cells is inefficient, and the remaining undifferentiated cells may form teratomas. This raises safety concerns for clinical applications of hiPSC-derived cellular products. To improve the safety of hiPSC, we attempted to site-specifically insert a herpes simplex virus 1 thymidine kinase (HSV1-TK) suicide gene at the endogenous OCT4 (POU5F1) locus of hiPSC. Since the endogenous OCT4 promoter is active in undifferentiated cells only, we speculated that the HSV1-TK suicide gene will be transcribed in undifferentiated cells only and that the remaining undifferentiated cells can be depleted by treating them with the prodrug ganciclovir (GCV) prior to transplantation. To insert the HSV1-TK gene at the OCT4 locus, we cotransfected hiPSC with a pair of plasmids encoding an OCT4-specific zinc finger nuclease (ZFN) and a donor plasmid harboring a promoter-less transgene cassette consisting of HSV1-TK and puromycin resistance gene sequences, flanked by OCT4 gene sequences. Puromycin resistant clones were established and characterized regarding their sensitivity to GCV and the site of integration of the HSV1-TK/puromycin resistance gene cassette. Of the nine puromycin-resistant iPSC clones analyzed, three contained the HSV1-TK transgene at the OCT4 locus, but they were not sensitive to GCV. The other six clones were GCV-sensitive, but the TK gene was located at off-target sites. These TK-expressing hiPSC clones remained GCV sensitive for up to 90 days, indicating that TK transgene expression was stable. Possible reasons for our failed attempt to selectively target the OCT4 locus are discussed.
PLoS ONE 2012 APR
Antibody-directed lentiviral gene transduction for live-cell monitoring and selection of human iPS and hES cells
Abstract
Abstract
The identification of stem cells within a mixed population of cells is a major hurdle for stem cell biology--in particular, in the identification of induced pluripotent stem (iPS) cells during the reprogramming process. Based on the selective expression of stem cell surface markers, a method to specifically infect stem cells through antibody-conjugated lentiviral particles has been developed that can deliver both visual markers for live-cell imaging as well as selectable markers to enrich for iPS cells. Antibodies recognizing SSEA4 and CD24 mediated the selective infection of the iPS cells over the parental human fibroblasts, allowing for rapid expansion of these cells by puromycin selection. Adaptation of the vector allows for the selective marking of human embryonic stem (hES) cells for their removal from a population of differentiated cells. This method has the benefit that it not only identifies stem cells, but that specific genes, including positive and negative selection markers, regulatory genes or miRNA can be delivered to the targeted stem cells. The ability to specifically target gene delivery to human pluripotent stem cells has broad applications in tissue engineering and stem cell therapies.
BioTechniques 2003
Rapid selection of Drosophila S2 cells with the puromycin resistance gene.
Abstract
Abstract
RNA (New York, N.Y.) 2000 MAY
Puromycin-rRNA interaction sites at the peptidyl transferase center.
Abstract
Abstract
The binding site of puromycin was probed chemically in the peptidyl-transferase center of ribosomes from Escherichia coli and of puromycin-hypersensitive ribosomes from the archaeon Haloferax gibbonsii. Several nucleotides of the 23S rRNAs showed altered chemical reactivities in the presence of puromycin. They include A2439, G2505, and G2553 for E. coli, and G2058, A2503, G2505, and G2553 for Hf. gibbonsii (using the E. coli numbering system). Reproducible enhanced reactivities were also observed at A508 and A1579 within domains I and III, respectively, of E. coli 23S rRNA. In further experiments, puromycin was shown to produce a major reduction in the UV-induced crosslinking of deacylated-(2N3A76)tRNA to U2506 within the P' site of E. coli ribosomes. Moreover, it strongly stimulated the putative UV-induced crosslink between a streptogramin B drug and m2A2503/psi2504 at an adjacent site in E. coli 23S rRNA. These data strongly support the concept that puromycin, along with other peptidyl-transferase antibiotics, in particular the streptogramin B drugs, bind to an RNA structural motif that contains several conserved and accessible base moieties of the peptidyl transferase loop region. This streptogramin motif is also likely to provide binding sites for the 3' termini of the acceptor and donor tRNAs. In contrast, the effects at A508 and A1579, which are located at the exit site of the peptide channel, are likely to be caused by a structural effect transmitted along the peptide channel.
1992
Recombinant DNA Part G
Abstract