Purmorphamine
Hedgehog pathway activator; Activates Smoothened (SMO)
概要
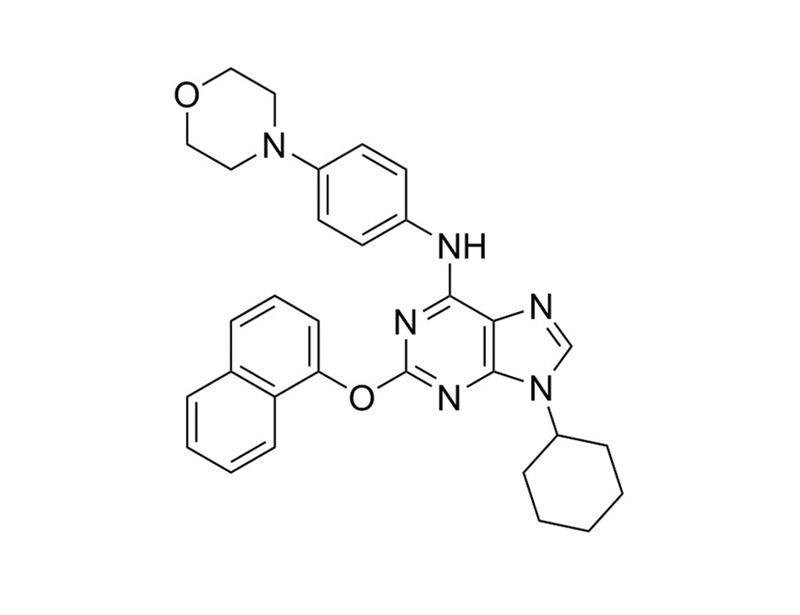
Purmorphamine is a tri-substituted purine derivative that activates the Hedgehog pathway by directly binding to and activating the Hedgehog receptor Smoothened (EC₅₀ = 1 µM). (Sinha and Chen)
DIFFERENTIATION
· Promotes differentiation of ventral spinal progenitors and motor neurons from human pluripotent stem cells (Hu and Zhang, Karumbayaram et al., Li et al.).
· Promotes differentiation of osteoblasts from human and mouse mesenchymal cells (Beloti et al., Wu et al. 2002, Wu et al. 2004).
· Inhibits differentiation and maturation of adipocytes from human mesenchymal cells (Fontaine et al.).
DIFFERENTIATION
· Promotes differentiation of ventral spinal progenitors and motor neurons from human pluripotent stem cells (Hu and Zhang, Karumbayaram et al., Li et al.).
· Promotes differentiation of osteoblasts from human and mouse mesenchymal cells (Beloti et al., Wu et al. 2002, Wu et al. 2004).
· Inhibits differentiation and maturation of adipocytes from human mesenchymal cells (Fontaine et al.).
Alternative Names
Not applicable
Cell Type
Mesenchymal Stem and Progenitor Cells, Neural Cells, PSC-Derived, Osteoblasts, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Neuroscience, Stem Cell Biology
CAS Number
483367-10-8
Chemical Formula
C₃₁H₃₂N₆O₂
Molecular Weight
520.6 g/mol
Purity
≥ 98%
Pathway
Hedgehog
Target
SMO
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Purmorphamine | 72202, 72204 | All | English |
| Safety Data Sheet | Purmorphamine | 72202, 72204 | All | English |
数据及文献
Publications (8)
Nature protocols 2009 JAN
Differentiation of spinal motor neurons from pluripotent human stem cells.
Abstract
Abstract
We have devised a reproducible protocol by which human embryonic stem cells (hESCs) or inducible pluripotent stem cells (iPSCs) are efficiently differentiated to functional spinal motor neurons. This protocol comprises four major steps. Pluripotent stem cells are induced to form neuroepithelial (NE) cells that form neural tube-like rosettes in the absence of morphogens in the first 2 weeks. The NE cells are then specified to OLIG2-expressing motoneuron progenitors in the presence of retinoic acid (RA) and sonic hedgehog (SHH) or purmorphamine in the next 2 weeks. These progenitor cells further generate post-mitotic, HB9-expressing motoneurons at the 5th week and mature to functional motor neurons thereafter. It typically takes 5 weeks to generate the post-mitotic motoneurons and 8-10 weeks for the production of functional mature motoneurons. In comparison with other methods, our protocol does not use feeder cells, has a minimum dependence on proteins (purmorphamine replacing SHH), has controllable adherent selection and is adaptable for scalable suspension culture.
Stem cells (Dayton, Ohio) 2009 APR
Directed differentiation of human-induced pluripotent stem cells generates active motor neurons.
Abstract
Abstract
The potential for directed differentiation of human-induced pluripotent stem (iPS) cells to functional postmitotic neuronal phenotypes is unknown. Following methods shown to be effective at generating motor neurons from human embryonic stem cells (hESCs), we found that once specified to a neural lineage, human iPS cells could be differentiated to form motor neurons with a similar efficiency as hESCs. Human iPS-derived cells appeared to follow a normal developmental progression associated with motor neuron formation and possessed prototypical electrophysiological properties. This is the first demonstration that human iPS-derived cells are able to generate electrically active motor neurons. These findings demonstrate the feasibility of using iPS-derived motor neuron progenitors and motor neurons in regenerative medicine applications and in vitro modeling of motor neuron diseases.
Stem cells (Dayton, Ohio) 2008 APR
Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules.
Abstract
Abstract
Specification of distinct cell types from human embryonic stem cells (hESCs) is key to the potential application of these naïve pluripotent cells in regenerative medicine. Determination of the nontarget differentiated populations, which is lacking in the field, is also crucial. Here, we show an efficient differentiation of motor neurons ( approximately 50%) by a simple sequential application of retinoid acid and sonic hedgehog (SHH) in a chemically defined suspension culture. We also discovered that purmorphamine, a small molecule that activates the SHH pathway, could replace SHH for the generation of motor neurons. Immunocytochemical characterization indicated that cells differentiated from hESCs were nearly completely restricted to the ventral spinal progenitor fate (NKX2.2+, Irx3+, and Pax7-), with the exception of motor neurons (HB9+) and their progenitors (Olig2+). Thus, the directed neural differentiation system with small molecules, even without further purification, will facilitate basic and translational studies using human motoneurons at a minimal cost.
Stem cells (Dayton, Ohio) 2008 APR
Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells.
Abstract
Abstract
Human stem cells are powerful tools by which to investigate molecular mechanisms of cell growth and differentiation under normal and pathological conditions. Hedgehog signaling, the dysregulation of which causes several pathologies, such as congenital defects and cancer, is involved in several cell differentiation processes and interferes with adipocyte differentiation of rodent cells. The present study was aimed at investigating the effect of Hedgehog pathway modulation on adipocyte phenotype using different sources of human mesenchymal cells, such as bone marrow stromal cells and human multipotent adipose-derived stem cells. We bring evidence that Hedgehog signaling decreases during human adipocyte differentiation. Inhibition of this pathway is not sufficient to trigger adipogenesis, but activation of Hedgehog pathway alters adipocyte morphology as well as insulin sensitivity. Analysis of glycerol-3-phosphate dehydrogenase activity and expression of adipocyte marker genes indicate that activation of Hedgehog signaling by purmorphamine impairs adipogenesis. In sharp contrast to reports in rodent cells, the maturation process, but not the early steps of human mesenchymal stem cell differentiation, is affected by Hedgehog activation. Hedgehog interferes with adipocyte differentiation by targeting CCAAT enhancer-binding protein alpha and peroxisome proliferator-activated receptor (PPAR) gamma2 expression, whereas PPARgamma1 level remains unaffected. Although Hedgehog pathway stimulation does not modify the total number of adipocytes, adipogenesis appears dramatically impaired, with reduced lipid accumulation, a decrease in adipocyte-specific markers, and acquisition of an insulin-resistant phenotype. This study indicates that a decrease in Hedgehog signaling is necessary but not sufficient to trigger adipocyte differentiation and unveils a striking difference in the adipocyte differentiation process between rodent and human mesenchymal stem cells.
Nature chemical biology 2006 JAN
Purmorphamine activates the Hedgehog pathway by targeting Smoothened.
Abstract
Abstract
Hedgehog (Hh) signaling is an important regulator of embryonic patterning, tissue regeneration, stem cell renewal and cancer growth. A purine derivative named purmorphamine was previously found to activate the Hh pathway and affect osteoblast differentiation through an unknown mechanism. We demonstrate here that purmorphamine directly targets Smoothened, a critical component of the Hh signaling pathway.
Cell biology international 2005 JUL
Purmorphamine enhances osteogenic activity of human osteoblasts derived from bone marrow mesenchymal cells.
Abstract
Abstract
Purmorphamine is a novel small molecule with osteogenesis-inducing activity in multipotent mesenchymal progenitor cells, but there has been no evaluation of its effect on human cells to date. The aim of this study was to investigate the induction of osteogenic activity by purmorphamine in human osteoblasts differentiated from bone marrow mesenchymal cells. Cells were cultured in 24-well plates at a density of 2x10(4)/well in medium containing 1, 2 or 3 microM purmorphamine, or vehicle. At 7, 14 and 21 days, cell proliferation, viability, and alkaline phosphatase (ALP) activity were evaluated. Bone-like nodule formation was evaluated at 21 days. Purmorphamine did not affect cell proliferation or viability, but increased ALP activity and bone-like nodule formation. These results indicate that events related to osteoblast differentiation, including increased ALP activity and bone-like nodule formation, are enhanced by purmorphamine.