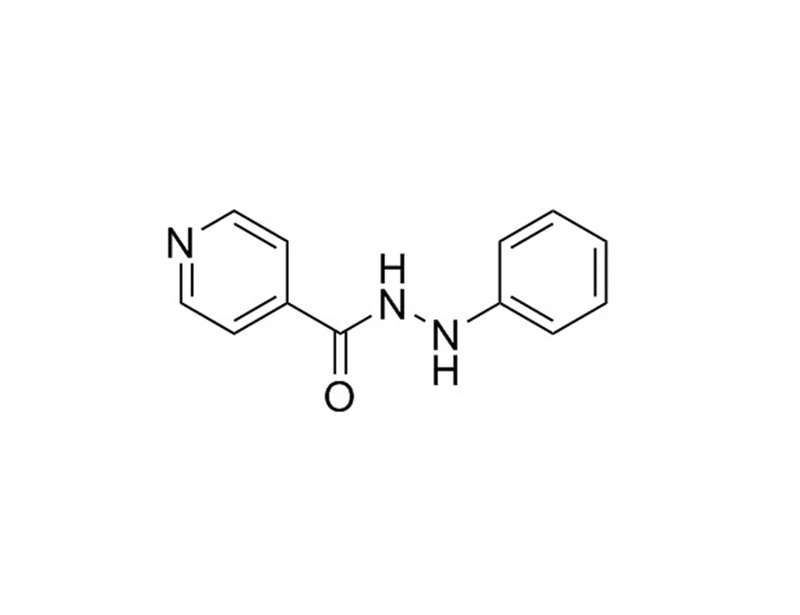
PluriSIn-1
Oleic acid biosynthesis pathway inhibitor; Inhibits stearoyl-CoA desaturase (SCD1)
概要
PluriSIn-1 is an N-acyl phenylhydrazine derivative that inhibits stearoyl-CoA desaturase, a key enzyme for lipid metabolism that is expressed in human pluripotent stem cells (Ben-David et al.).
DIFFERENTIATION
· Selectively eliminates undifferentiated human embryonic stem (ES) and induced pluripotent stem (iPS) cells while sparing differentiated cells, and prevents teratoma formation in transplanted mice (Ben-David et al.).
· Induces apoptosis of Nanog-positive iPS cells in vitro, while leaving iPS cell-derived cardiomyocytes unaffected (Zhang et al.).
DIFFERENTIATION
· Selectively eliminates undifferentiated human embryonic stem (ES) and induced pluripotent stem (iPS) cells while sparing differentiated cells, and prevents teratoma formation in transplanted mice (Ben-David et al.).
· Induces apoptosis of Nanog-positive iPS cells in vitro, while leaving iPS cell-derived cardiomyocytes unaffected (Zhang et al.).
Alternative Names
N’-phenyl-hydrazine-Isonicotinic acid; NSC 14613
Cell Type
Cardiomyocytes, PSC-Derived, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Stem Cell Biology
CAS Number
91396-88-2
Chemical Formula
C₁₂H₁₁N₃O
Molecular Weight
213.2 g/mol
Purity
≥ 95%
Pathway
Oleic Acid
Target
SCD1
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | PluriSIn-1 | 72822, 72824 | All | English |
| Safety Data Sheet | PluriSIn-1 | 72822, 72824 | All | English |
数据及文献
Publications (2)
Cell cycle (Georgetown, Tex.) 2014 MAR
Inhibition of stearoyl-coA desaturase selectively eliminates tumorigenic Nanog-positive cells: improving the safety of iPS cell transplantation to myocardium.
Abstract
Abstract
Induced pluripotent stem cells (iPS) can differentiate into cardiomyocytes (CM) and represent a promising form of cellular therapy for heart regeneration. However, residual undifferentiated iPS derivates (iPSD), which are not fully eliminated by cell differentiation or purification protocols, may form tumors after transplantation, thus compromising therapeutic application. Inhibition of stearoyl-coA desaturase (SCD) has recently been reported to eliminate undifferentiated human embryonic stem cells, which share many features with iPSD. Here, we tested the effects of PluriSin1, a small-molecule inhibitor of SCD, on iPS-derived CM. We found that plurisin1 treatment significantly decreased the mRNA and protein level of Nanog, a marker for both cell pluripotency and tumor progression; importantly, we provide evidence that PluriSin1 treatment at 20 µM for 1 day significantly induces the apoptosis of Nanog-positive iPSD. In addition, PluriSin1 treatment at 20 µM for 4 days diminished Nanog-positive stem cells in cultured iPSD while not increasing apoptosis of iPS-derived CM. To investigate whether PluriSin1 treatment prevents tumorigenicity of iPSD after cell transplantation, we intramyocardially injected PluriSin1- or DMSO-treated iPSD in a mouse model of myocardial infarction (MI). DMSO-treated iPSD readily formed Nanog-expressing tumors 2 weeks after injection, which was prevented by treatment with PluriSin1. Moreover, treatment with PluriSin1 did not change the expression of cTnI, α-MHC, or MLC-2v, markers of cardiac differentiation (Ptextgreater0.05, n = 4). Importantly, pluriSin1-treated iPS-derived CM exhibited the ability to engraft and survive in the infarcted myocardium. We conclude that inhibition of SCD holds the potential to enhance the safety of therapeutic application of iPS cells for heart regeneration.
Cell stem cell 2013 FEB
Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen
Abstract
Abstract
The use of human pluripotent stem cells (hPSCs) in cell therapy is hindered by the tumorigenic risk from residual undifferentiated cells. Here we performed a high-throughput screen of over 52,000 small molecules and identified 15 pluripotent cell-specific inhibitors (PluriSIns), nine of which share a common structural moiety. The PluriSIns selectively eliminated hPSCs while sparing a large array of progenitor and differentiated cells. Cellular and molecular analyses demonstrated that the most selective compound, PluriSIn 1, induces ER stress, protein synthesis attenuation, and apoptosis in hPSCs. Close examination identified this molecule as an inhibitor of stearoyl-coA desaturase (SCD1), the key enzyme in oleic acid biosynthesis, revealing a unique role for lipid metabolism in hPSCs. PluriSIn 1 was also cytotoxic to mouse blastocysts, indicating that the dependence on oleate is inherent to the pluripotent state. Finally, application of PluriSIn 1 prevented teratoma formation from tumorigenic undifferentiated cells. These findings should increase the safety of hPSC-based treatments. ?? 2013 Elsevier Inc.