Pifithrin-mu
p53 inhibitor
概要
Pifithrin-mu (PFT-µ) is an inhibitor of p53-mediated apoptosis, preventing p53 binding to Bcl-xL and Bcl-2 at the mitochondrial surface, without affecting p53 transactivational activities (Strom et al.). In vitro, PFT-µ binds both p53 (Kd = 0.82 mM) and Bcl-xL (Kd = 0.80 mM; Hagn et al.).
MAINTENANCE AND SELF-RENEWAL
· In combination with Rho-associated coiled-coil containing protein kinase (ROCK) inhibitor Y-27632, improves cell recovery after cryopreservation (Xu et al.).
· Inhibits DNA damage-induced apoptosis in human embryonic stem cells (Qin et al.).
MAINTENANCE AND SELF-RENEWAL
· In combination with Rho-associated coiled-coil containing protein kinase (ROCK) inhibitor Y-27632, improves cell recovery after cryopreservation (Xu et al.).
· Inhibits DNA damage-induced apoptosis in human embryonic stem cells (Qin et al.).
Alternative Names
2-Phenylethynesulfonamide; PFT-μ; Pifithrin-μ
Cell Type
Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Maintenance
Area of Interest
Stem Cell Biology
CAS Number
64984-31-2
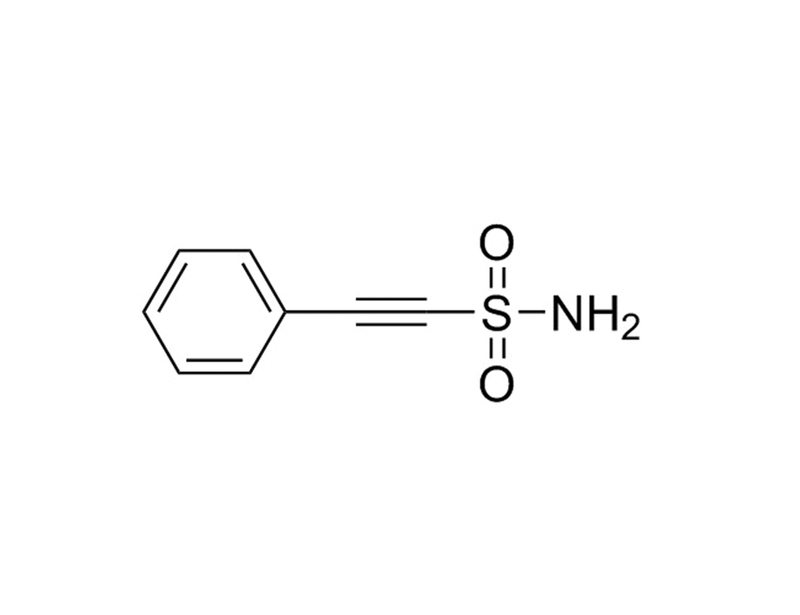
Chemical Formula
C₈H₇NO₂S
Molecular Weight
181.2 g/mol
Purity
≥ 98%
Pathway
p53
Target
p53
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Pifithrin-mu | 72802, 72804 | All | English |
| Safety Data Sheet | Pifithrin-mu | 72802, 72804 | All | English |
数据及文献
Publications (5)
The Journal of biological chemistry 2010 JAN
BclxL changes conformation upon binding to wild-type but not mutant p53 DNA binding domain.
Abstract
Abstract
p53 can induce apoptosis through mitochondrial membrane permeabilization by interaction of its DNA binding region with the anti-apoptotic proteins BclxL and Bcl2. However, little is known about the action of p53 at the mitochondria in molecular detail. By using NMR spectroscopy and fluorescence polarization we characterized the binding of wild-type and mutant p53 DNA binding domains to BclxL and show that the wild-type p53 DNA binding domain leads to structural changes in the BH3 binding region of BclxL, whereas mutants fail to induce such effects due to reduced affinity. This was probed by induced chemical shift and residual dipolar coupling data. These data imply that p53 partly achieves its pro-apoptotic function at the mitochondria by facilitating interaction between BclxL and BH3-only proteins in an allosteric mode of action. Furthermore, we characterize for the first time the binding behavior of Pifithrin-mu, a specific small molecule inhibitor of the p53-BclxL interaction, and present a structural model of the protein-ligand complex. A rather unusual behavior is revealed whereby Pifithrin-mu binds to both sides of the protein-protein complex. These data should facilitate the rational design of more potent specific BclxL-p53 inhibitors.
Biotechnology progress 2010
Enhancement of cell recovery for dissociated human embryonic stem cells after cryopreservation.
Abstract
Abstract
Due to widespread applications of human embryonic stem (hES) cells, it is essential to establish effective protocols for cryopreservation and subsequent culture of hES cells to improve cell recovery. We have developed a new protocol for cryopreservation of dissociated hES cells and subsequent culture. We examined the effects of new formula of freezing solution containing 7.5% dimethylsulfoxide (DMSO) (v/v %) and 2.5% polyethylene glycol (PEG) (w/v %) on cell survival and recovery of hES cells after cryopreservation, and further investigated the role of the combination of Rho-associated kinase (ROCK) inhibitor and p53 inhibitor on cell recovery during the subsequent culture. Compared with the conventional slow-freezing method which uses 10% DMSO as a freezing solution and then cultured in the presence of ROCK inhibitor at the first day of culture, we found out that hES cell recovery was significantly enhanced by around 30 % (P textless 0.05) by the new freezing solution. Moreover, at the first day of post-thaw culture, the presence of 10 microM ROCK inhibitor (Y-27632) and 1 microM pifithrin-mu together further significantly improved cell recovery by around 20% (P textless 0.05) either for feeder-dependent or feeder-independent culture. hES cells remained their undifferentiated status after using this novel protocol for cryopreservation and subsequent culture. Furthermore, this protocol is a scalable cryopreservation method for handling large quantities of hES cells.
Molecular cell 2009 OCT
A small molecule inhibitor of inducible heat shock protein 70.
Abstract
Abstract
The multifunctional, stress-inducible molecular chaperone HSP70 has important roles in aiding protein folding and maintaining protein homeostasis. HSP70 expression is elevated in many cancers, contributing to tumor cell survival and resistance to therapy. We have determined that a small molecule called 2-phenylethynesulfonamide (PES) interacts selectively with HSP70 and leads to a disruption of the association between HSP70 and several of its cochaperones and substrate proteins. Treatment of cultured tumor cells with PES promotes cell death that is associated with protein aggregation, impaired autophagy, and inhibition of lysosomal function. Moreover, this small molecule is able to suppress tumor development and enhance survival in a mouse model of Myc-induced lymphomagenesis. The data demonstrate that PES disrupts actions of HSP70 in multiple cell signaling pathways, offering an opportunity to better understand the diverse functions of this molecular chaperone and also to aid in the development of new cancer therapies.
The Journal of biological chemistry 2007 FEB
Regulation of apoptosis and differentiation by p53 in human embryonic stem cells.
Abstract
Abstract
The essentially infinite expansion potential and pluripotency of human embryonic stem cells (hESCs) makes them attractive for cell-based therapeutics. In contrast to mouse embryonic stem cells (mESCs), hESCs normally undergo high rates of spontaneous apoptosis and differentiation, making them difficult to maintain in culture. Here we demonstrate that p53 protein accumulates in apoptotic hESCs induced by agents that damage DNA. However, despite the accumulation of p53, it nevertheless fails to activate the transcription of its target genes. This inability of p53 to activate its target genes has not been observed in other cell types, including mESCs. We further demonstrate that p53 induces apoptosis of hESCs through a mitochondrial pathway. Reducing p53 expression in hESCs in turn reduces both DNA damage-induced apoptosis as well as spontaneous apoptosis. Reducing p53 expression also reduces spontaneous differentiation and slows the differentiation rate of hESCs. Our studies reveal the important roles of p53 as a critical mediator of human embryonic stem cells survival and differentiation.
Nature chemical biology 2006 SEP
Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation.
Abstract
Abstract
p53-dependent apoptosis contributes to the side effects of cancer treatment, and genetic or pharmacological inhibition of p53 function can increase normal tissue resistance to genotoxic stress. It has recently been shown that p53 can induce apoptosis through a mechanism that does not depend on transactivation but instead involves translocation of p53 to mitochondria. To determine the impact of this p53 activity on normal tissue radiosensitivity, we isolated a small molecule named pifithrin-mu (PFTmu, 1) that inhibits p53 binding to mitochondria by reducing its affinity to antiapoptotic proteins Bcl-xL and Bcl-2 but has no effect on p53-dependent transactivation. PFTmu has a high specificity for p53 and does not protect cells from apoptosis induced by overexpression of proapoptotic protein Bax or by treatment with dexamethasone (2). PFTmu rescues primary mouse thymocytes from p53-mediated apoptosis caused by radiation and protects mice from doses of radiation that cause lethal hematopoietic syndrome. These results indicate that selective inhibition of the mitochondrial branch of the p53 pathway is sufficient for radioprotection in vivo.