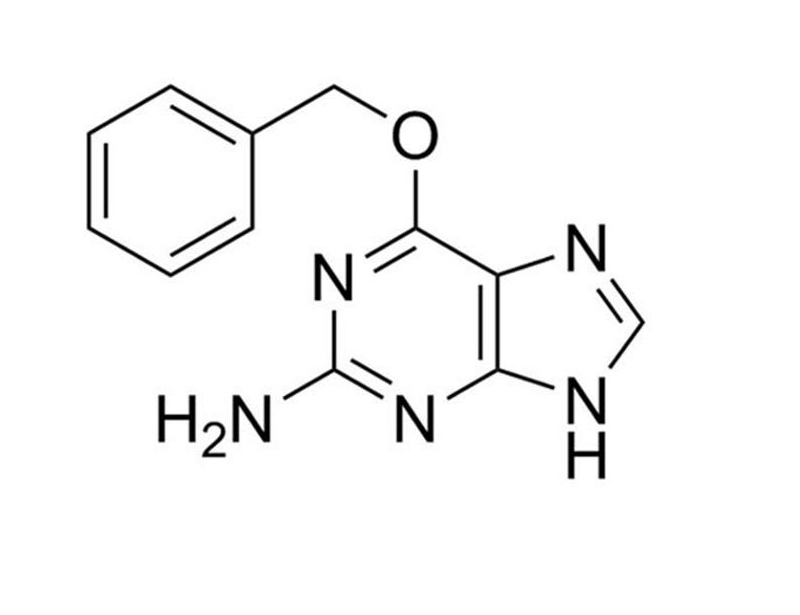
O6-Benzylguanine
Epigenetic modifier; Inactivates O6-alkylguanine-DNA Alkyltransferase (AGT)
概要
O6-Benzylguanine is an efficient irreversible inactivator of the DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT, also known as methylguanine DNA methyltransferase, or MGMT). AGT directly removes alkyl groups located on the O6-position of guanine from DNA, thereby restoring DNA integrity. O6-Benzylguanine is an antineoplastic agent that can be used to investigate the role of AGT in carcinogenesis and mutagenesis (Pegg 2011; Dolan et al.).
CANCER RESEARCH
· Enhances the activity of alkylating agents (nitrosourea, temozolomide, and cyclophosphamide) in malignant glioma xenografts growing in athymic nude mice (Pegg 1990).
· Sensitizes CD34+ hematopoietic progenitors and a breast cancer cell line to bis-chloroethylnitrosourea (BCNU; Gerson et al.).
CANCER RESEARCH
· Enhances the activity of alkylating agents (nitrosourea, temozolomide, and cyclophosphamide) in malignant glioma xenografts growing in athymic nude mice (Pegg 1990).
· Sensitizes CD34+ hematopoietic progenitors and a breast cancer cell line to bis-chloroethylnitrosourea (BCNU; Gerson et al.).
Alternative Names
NSC 637037
Cell Type
Cancer Cells and Cell Lines, Hematopoietic Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research
CAS Number
19916-73-5
Chemical Formula
C₁₂H₁₁N₅O
Molecular Weight
241.2 g/mol
Purity
≥ 98%
Pathway
Epigenetic
Target
AGT
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | O6-Benzylguanine | 73762 | All | English |
| Safety Data Sheet | O6-Benzylguanine | 73762 | All | English |
数据及文献
Publications (4)
Chemical research in toxicology 2011 MAY
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools.
Abstract
Abstract
O(6)-Alkylguanine-DNA alkyltransferase (AGT) is a widely distributed, unique DNA repair protein that acts as a single agent to directly remove alkyl groups located on the O(6)-position of guanine from DNA restoring the DNA in one step. The protein acts only once, and its alkylated form is degraded rapidly. It is a major factor in counteracting the mutagenic, carcinogenic, and cytotoxic effects of agents that form such adducts including N-nitroso-compounds and a number of cancer chemotherapeutics. This review describes the structure, function, and mechanism of action of AGTs and of a family of related alkyltransferase-like proteins, which do not act alone to repair O(6)-alkylguanines in DNA but link repair to other pathways. The paradoxical ability of AGTs to stimulate the DNA-damaging ability of dihaloalkanes and other bis-electrophiles via the formation of AGT-DNA cross-links is also described. Other important properties of AGTs include the ability to provide resistance to cancer therapeutic alkylating agents, and the availability of AGT inhibitors such as O(6)-benzylguanine that might overcome this resistance is discussed. Finally, the properties of fusion proteins in which AGT sequences are linked to other proteins are outlined. Such proteins occur naturally, and synthetic variants engineered to react specifically with derivatives of O(6)-benzylguanine are the basis of a valuable research technique for tagging proteins with specific reagents.
Blood 1996 SEP
Human CD34+ hematopoietic progenitors have low, cytokine-unresponsive O6-alkylguanine-DNA alkyltransferase and are sensitive to O6-benzylguanine plus BCNU.
Abstract
Abstract
Human bone marrow (BM) cells contain low levels of the DNA repair protein, O6-alkylguanine-DNA alkyltransferase, which may explain their susceptibility to nitrosourea-induced cytotoxicity and the development of secondary leukemia after nitrosourea treatment. Isolated CD34+ myeloid progenitors were also found to have low levels of alkyltransferase activity. The level of alkyltransferase in CD34+ cells or in mononuclear BM cells did not increase after incubation with granulocyte-macrophage colony-stimulating factor, interleukin-3, stem cell factor, the combination, or 5637 conditioned medium. BCNU sensitivity remained unchanged as well. In addition, O6-benzylguanine depleted alkyltransferase activity in BM cells at concentrations as low as 1.5 mumol/L after a 1-hour exposure. O6-benzylguanine pretreatment markedly sensitized hematopoietic progenitor colony-forming cells to BCNU, resulting in a reduction in the dose of drug (termed the dose-modification factor) required to inhibit 50% of the colony formation (IC50) of threefold to fivefold. Since, unlike many other cell types, proliferating early (CD34+) hematopoietic precursors do not induce alkyltransferase, myelosuppression may be the dose-limiting toxicity of the combination of O6-benzylguanine plus BCNU in clinical trials.
Cancer research 1990 OCT
Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents.
Abstract
Abstract
Proceedings of the National Academy of Sciences of the United States of America 1990 JUL
Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents.
Abstract
Abstract
O6-Alkylguanine-DNA alkyltransferase was rapidly and irreversibly inactivated by exposure to O6-benzylguanine or the p-chlorobenzyl and p-methylbenzyl analogues. This inactivation was much more rapid than with O6-methylguanine: incubation with 2.5 microM O6-benzylguanine led to more than a 90% loss of activity within 10 min, whereas 0.2 mM O6-methylguanine for 60 min was required for the same reduction. O6-Benzylguanine was highly effective in depleting the alkyltransferase activity of cultured human colon tumor (HT29) cells. Complete loss of activity was produced within 15 min after addition of O6-benzylguanine to the culture medium and a maximal effect was obtained with 5 microM. In contrast, at least 100 microM O6-methylguanine for 4 hr was needed to get a maximal effect, and this reduced the alkyltransferase by only 80%. Pretreatment of HT29 cells with 10 microM O6-benzylguanine for 2 hr led to a dramatic increase in the cytotoxicity produced by the chemotherapeutic agents 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) or 2-chloroethyl(methysulfonyl)methanesulfonate (Clomesone). Administration of O6-benzylguanine to mice at a dose of 10 mg/kg reduced alkyltransferase levels by more than 95% in both liver and kidney. These results indicate that depletion of the alkyltransferase by O6-benzylguanine may be used to investigate the role of the DNA repair protein in carcinogenesis and mutagenesis and that this treatment may be valuable to increase the chemotherapeutic effectiveness of chloroethylating agents.