Mitomycin C
Antibiotic; Double-stranded DNA alkylating agent
概要
Mitomycin C is an antibiotic which acts as a double-stranded DNA alkylating agent. It covalently crosslinks DNA, inhibiting DNA synthesis and cell proliferation. It acts by way of reductive activation either through low pH or NAD(P)H:quinone oxidoreductase (DT-diaphorase) or NADH cytochrome c reductase (Mao et al.; Cummings et al.).
MAINTENANCE
· Mitotically inactivates mouse embryonic fibroblasts (MEFs) for use as feeder cell layers in embryonic stem cell co-culture systems (Bryja et al.).
CANCER RESEARCH
· Selectively inhibits DNA synthesis and mutagenesis, stimulates genetic recombination, chromosome breakage and sister chromatid exchange, and induces DNA repair (Tomasz).
MAINTENANCE
· Mitotically inactivates mouse embryonic fibroblasts (MEFs) for use as feeder cell layers in embryonic stem cell co-culture systems (Bryja et al.).
CANCER RESEARCH
· Selectively inhibits DNA synthesis and mutagenesis, stimulates genetic recombination, chromosome breakage and sister chromatid exchange, and induces DNA repair (Tomasz).
Alternative Names
Ametycine; MitoExtra; Mitonco; Mitoplus; MMC; NSC 26980
Cell Type
Cancer Cells and Cell Lines, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Antibiotic, Maintenance
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
50-07-7
Chemical Formula
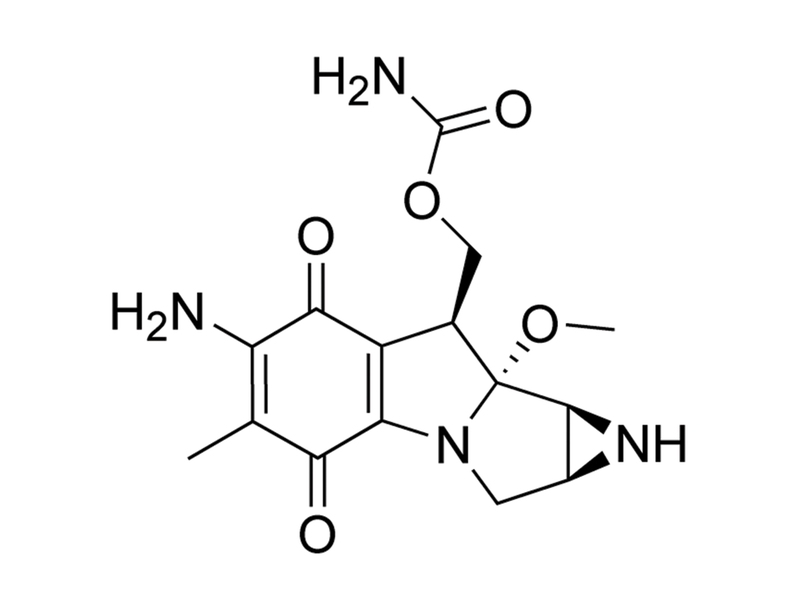
C₁₅H₁₈N₄O₅
Molecular Weight
334.3 g/mol
Purity
≥ 98%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Mitomycin C | 73272, 73274 | All | English |
| Safety Data Sheet | Mitomycin C | 73272, 73274 | All | English |
数据及文献
Publications (4)
Nature protocols 2006
Derivation of mouse embryonic stem cells.
Abstract
Abstract
Here we describe a simple and efficient protocol for derivation of germline chimera-competent mouse embryonic stem cells (mESCs) from embryonic day 3.5 (E3.5) blastocysts. The protocol involves the use of early-passage mouse embryonic fibroblast feeders (MEF) and the alternation of fetal bovine serum- and serum replacement (SR)-containing media. As compared to other available protocols for mESCs derivation, our protocol differs in the combination of commercial availability of all reagents, technical simplicity and high efficiency. mESC lines are derived with approximately 50% efficiency (50 independent mESC lines derived from 96 blastocysts). We believe that this protocol could be a good starting point for (i) setting up the derivation of mESC lines in a laboratory and (ii) incorporating further steps to improve efficiency or adapt the protocol to other applications. The whole process (from blastocyst extraction to the freezing of mESC line) usually takes between 15 and 20 d.
Chemistry & biology 1999
Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564.
Abstract
Abstract
BACKGROUND: The mitomycins are natural products that contain a variety of functional groups, including aminobenzoquinone- and aziridine-ring systems. Mitomycin C (MC) was the first recognized bioreductive alkylating agent, and has been widely used clinically for antitumor therapy. Precursor-feeding studies showed that MC is derived from 3-amino-5-hydroxybenzoic acid (AHBA), D-glucosamine, L-methionine and carbamoyl phosphate. A genetically linked AHBA biosynthetic gene and MC resistance genes were identified previously in the MC producer Streptomyces lavendulae NRRL 2564. We set out to identify other genes involved in MC biosynthesis. RESULTS: A cluster of 47 genes spanning 55 kilobases of S. lavendulae DNA governs MC biosynthesis. Fourteen of 22 disruption mutants did not express or overexpressed MC. Seven gene products probably assemble the AHBA intermediate through a variant of the shikimate pathway. The gene encoding the first presumed enzyme in AHBA biosynthesis is not, however, linked within the MC cluster. Candidate genes for mitosane nucleus formation and functionalization were identified. A putative MC translocase was identified that comprises a novel drug-binding and export system, which confers cellular self-protection on S. lavendulae. Two regulatory genes were also identified. CONCLUSIONS: The overall architecture of the MC biosynthetic gene cluster in S. lavendulae has been determined. Targeted manipulation of a putative MC pathway regulator led to a substantial increase in drug production. The cloned genes should help elucidate the molecular basis for creation of the mitosane ring system, as well efforts to engineer the biosynthesis of novel natural products.
Biochemical pharmacology 1998
Enzymology of mitomycin C metabolic activation in tumour tissue: implications for enzyme-directed bioreductive drug development.
Abstract
Abstract
Mitomycin C (MMC) is the prototype bioreductive DNA alkylating agent. To exploit its unique properties and maximize patient responses, different therapeutic approaches have been investigated. Recently, the focus has concentrated on monitoring the levels of the proteins metabolizing the drug and relating these to activity in a regimen referred to as enzyme-directed bioreductive drug development. To be successful, it is important to understand the enzymology of metabolic activation not only in cell lines but also in solid tumour models. A general mechanism of action for MMC has now emerged that is activated regardless of the source of reducing equivalents, comprising three competing pathways that give rise to unique reactive intermediates and different DNA adducts. Partitioning into the pathways is dictated by chemical considerations such as pH and drug concentration. DT-diaphorase stands out in this mechanism, since it is much less effective at metabolizing MMC at neutral pH. At least five different enzymes can catalyse MMC bioreduction in vitro, and as many activities may be present in solid tumours, including a series of novel mitochondrial reductases such as a cytochrome P450 reductase. Competition between reductases for MMC appears to be based solely on protein levels rather than enzyme kinetics. Consequentially, DT-diaphorase can occupy a central role in MMC metabolic activation since it is often highly overexpressed in cancer cells. Although a good correlation has been observed in cell lines between DT-diaphorase expression and aerobic cytotoxicity, this does not hold consistently in vivo for any single bioreductive enzyme, suggesting revision of the enzyme-directed hypothesis as originally formulated.
Chemistry & biology 1995
Mitomycin C: small, fast and deadly (but very selective).
Abstract
Abstract
Mitomycin C, an important antitumor drug and antibiotic, has an extraordinary ability to crosslink DNA with high efficiency and absolute specificity for the sequence CpG. Recent results have shown how mitomycin C crosslinks DNA, and why the sequence specificity is so complete. This new understanding may allow the design of agents that mimic mitomycin C's economy of structure and can crosslink other sequences.