Metformin
AMPK activator; Mitochondrial respiratory chain complex 1 inhibitor
概要
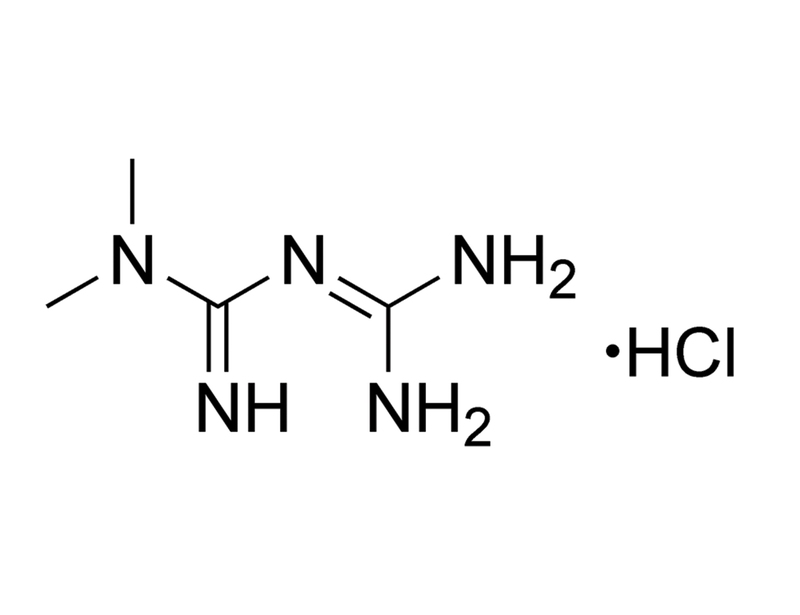
Metformin is an activator of the AMP-activated protein kinase (AMPK) pathway, and an inhibitor of mitochondrial respiratory chain complex 1 (Rena et al.; Viollet et al.). It acts as an antihyperglycemic agent to lower plasma glucose levels and improve insulin sensitivity (Viollet et al.). This product is provided as a hydrochloride salt of the molecule.
DIFFERENTIATION
· Promotes neurogenesis in mouse cortical precursors and human forebrain neural precursors in vitro, and in adult mouse central nervous system in vivo, via activation of the aPKC-CBP pathway (Wang et al.).
METABOLISM
· Stimulates glucose uptake in skeletal muscle and suppresses gluconeogenesis in the liver (Kim et al.; Shaw et al.).
· Reduces fatty liver disease in obese (ob/ob) mice (Lin et al.).
· Inhibits secretion of the adipocyte hormone leptin in mouse brown adipocytes (Klein et al.).
CANCER RESEARCH
· Inhibits tumor cell growth in various cancer cell lines and in mouse xenograft models (Dowling et al.; Zakikhani et al.; Isakovic et al.).
· Inhibits the inflammatory response associated with cancer stem cell growth (Hirsch et al.).
DIFFERENTIATION
· Promotes neurogenesis in mouse cortical precursors and human forebrain neural precursors in vitro, and in adult mouse central nervous system in vivo, via activation of the aPKC-CBP pathway (Wang et al.).
METABOLISM
· Stimulates glucose uptake in skeletal muscle and suppresses gluconeogenesis in the liver (Kim et al.; Shaw et al.).
· Reduces fatty liver disease in obese (ob/ob) mice (Lin et al.).
· Inhibits secretion of the adipocyte hormone leptin in mouse brown adipocytes (Klein et al.).
CANCER RESEARCH
· Inhibits tumor cell growth in various cancer cell lines and in mouse xenograft models (Dowling et al.; Zakikhani et al.; Isakovic et al.).
· Inhibits the inflammatory response associated with cancer stem cell growth (Hirsch et al.).
Alternative Names
Apophage; Diaformin; Fornidd; Glucoformin; Glucophage; LA 6023; Melbin; Orabet; Riomet; Walaphage
Cell Type
Adipocytes, Cancer Cells and Cell Lines, Myogenic Stem and Progenitor Cells, Neural Stem and Progenitor Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Cancer Research, Epithelial Cell Biology, Immunology, Metabolism, Neuroscience
CAS Number
1115-70-4
Chemical Formula
C₄H₁₁N₅ · HCl
Molecular Weight
165.6 g/mol
Purity
≥ 98%
Pathway
Mitochondrial Respiratory Chain Complex, AMPK
Target
AMPK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Metformin (Hydrochloride) | 73252, 73254 | All | English |
| Safety Data Sheet | Metformin (Hydrochloride) | 73252, 73254 | All | English |
数据及文献
Publications (13)
Proceedings of the National Academy of Sciences of the United States of America 2013 JAN
Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth.
Abstract
Abstract
Metformin, the first-line drug for treating diabetes, inhibits cellular transformation and selectively kills cancer stem cells in breast cancer cell lines. In a Src-inducible model of cellular transformation, metformin inhibits the earliest known step in the process, activation of the inflammatory transcription factor NF-κB. Metformin strongly delays cellular transformation in a manner similar to that occurring upon a weaker inflammatory stimulus. Conversely, inhibition of transformation does not occur if metformin is added after the initial inflammatory stimulus. The antitransformation effect of metformin can be bypassed by overexpression of Lin28B or IL1β, downstream targets of NF-κB. Metformin preferentially inhibits nuclear translocation of NF-κB and phosphorylation of STAT3 in cancer stem cells compared with non-stem cancer cells in the same population. The ability of metformin to block tumor growth and prolong remission in xenografts in combination with doxorubicin is associated with decreased function of the inflammatory feedback loop. Lastly, metformin-based combinatorial therapy is effective in xenografts involving inflammatory prostate and melanoma cell lines, whereas it is ineffective in noninflammatory cell lines from these lineages. Taken together, our observations suggest that metformin inhibits a signal transduction pathway that results in an inflammatory response. As metformin alters energy metabolism in diabetics, we speculate that metformin may block a metabolic stress response that stimulates the inflammatory pathway associated with a wide variety of cancers.
Diabetologia 2013
Molecular mechanism of action of metformin: old or new insights?
Abstract
Abstract
Metformin is the first-line drug treatment for type 2 diabetes. Globally, over 100 million patients are prescribed this drug annually. Metformin was discovered before the era of target-based drug discovery and its molecular mechanism of action remains an area of vigorous diabetes research. An improvement in our understanding of metformin's molecular targets is likely to enable target-based identification of second-generation drugs with similar properties, a development that has been impossible up to now. The notion that 5' AMP-activated protein kinase (AMPK) mediates the anti-hyperglycaemic action of metformin has recently been challenged by genetic loss-of-function studies, thrusting the AMPK-independent effects of the drug into the spotlight for the first time in more than a decade. Key AMPK-independent effects of the drug include the mitochondrial actions that have been known for many years and which are still thought to be the primary site of action of metformin. Coupled with recent evidence of AMPK-independent effects on the counter-regulatory hormone glucagon, new paradigms of AMPK-independent drug action are beginning to take shape. In this review we summarise the recent research developments on the molecular action of metformin.
Cell cycle (Georgetown, Tex.) 2012 MAR
Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells.
Abstract
Abstract
The ability of somatic cells to reprogram their ATP-generating machinery into a Warburg-like glycolytic metabotype while overexpressing stemness genes facilitates their conversion into either induced pluripotent stem cells (iPSCs) or tumor-propagating cells. AMP-activated protein kinase (AMPK) is a metabolic master switch that senses and decodes intracellular changes in energy status; thus, we have evaluated the impact of AMPK activation in regulating the generation of iPSCs from nonstem cells of somatic origin. The indirect and direct activation of AMPK with the antidiabetic biguanide metformin and the thienopyridone A-769662, respectively, impeded the reprogramming of mouse embryonic and human diploid fibroblasts into iPSCs. The AMPK activators established a metabolic barrier to reprogramming that could not be bypassed, even through p53 deficiency, a fundamental mechanism to greatly improve the efficiency of stem-cell production. Treatment with metformin or A-769662 before the generation of iPSC colonies was sufficient to drastically decrease iPSC generation, suggesting that AMPK activation impedes early stem cell genetic reprogramming. Monitoring the transcriptional activation status of each individual reprogramming factor (i.e., Oct4, Sox2, Klf4 and c-Myc) revealed that AMPK activation notably prevented the transcriptional activation of Oct4, the master regulator of the pluripotent state. AMPK activation appears to impose a normalized metabolic flow away from the required pro-immortalizing glycolysis that fuels the induction of stemness and pluripotency, endowing somatic cells with an energetic infrastructure that is protected against reprogramming. AMPK-activating anti-reprogramming strategies may provide a roadmap for the generation of novel cancer therapies that metabolically target tumor-propagating cells.
Journal of molecular endocrinology 2012
Metformin in cancer: translational challenges.
Abstract
Abstract
The anti-diabetic drug metformin is rapidly emerging as a potential anti-cancer agent. Metformin, effective in treating type 2 diabetes and the insulin resistance syndromes, improves insulin resistance by reducing hepatic gluconeogenesis and by enhancing glucose uptake by skeletal muscle. Epidemiological studies have consistently associated metformin use with decreased cancer incidence and cancer-related mortality. Furthermore, numerous preclinical and clinical studies have demonstrated anti-cancer effects of metformin, leading to an explosion of interest in evaluating this agent in human cancer. The effects of metformin on circulating insulin levels indicate a potential efficacy towards cancers associated with hyperinsulinaemia; however, metformin may also directly inhibit tumour growth. In this review, we describe the mechanism of action of metformin and summarise the epidemiological, clinical and preclinical evidence supporting a role for metformin in the treatment of cancer. In addition, the challenges associated with translating preclinical results into therapeutic benefit in the clinical setting will be discussed.
Cell stem cell 2012
Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation.
Abstract
Abstract
VIDEO ABSTRACT: Although endogenous recruitment of adult neural stem cells has been proposed as a therapeutic strategy, clinical approaches for achieving this are lacking. Here, we show that metformin, a widely used drug, promotes neurogenesis and enhances spatial memory formation. Specifically, we show that an atypical PKC-CBP pathway is essential for the normal genesis of neurons from neural precursors and that metformin activates this pathway to promote rodent and human neurogenesis in culture. Metformin also enhances neurogenesis in the adult mouse brain in a CBP-dependent fashion, and in so doing enhances spatial reversal learning in the water maze. Thus, metformin, by activating an aPKC-CBP pathway, recruits neural stem cells and enhances neural function, thereby providing a candidate pharmacological approach for nervous system therapy.
Clinical science (London, England : 1979) 2012
Cellular and molecular mechanisms of metformin: an overview.
Abstract
Abstract
Considerable efforts have been made since the 1950s to better understand the cellular and molecular mechanisms of action of metformin, a potent antihyperglycaemic agent now recommended as the first-line oral therapy for T2D (Type 2 diabetes). The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory chain complex I. In addition, the resulting decrease in hepatic energy status activates AMPK (AMP-activated protein kinase), a cellular metabolic sensor, providing a generally accepted mechanism for the action of metformin on hepatic gluconeogenesis. The demonstration that respiratory chain complex I, but not AMPK, is the primary target of metformin was recently strengthened by showing that the metabolic effect of the drug is preserved in liver-specific AMPK-deficient mice. Beyond its effect on glucose metabolism, metformin has been reported to restore ovarian function in PCOS (polycystic ovary syndrome), reduce fatty liver, and to lower microvascular and macrovascular complications associated with T2D. Its use has also recently been suggested as an adjuvant treatment for cancer or gestational diabetes and for the prevention in pre-diabetic populations. These emerging new therapeutic areas for metformin will be reviewed together with recent findings from pharmacogenetic studies linking genetic variations to drug response, a promising new step towards personalized medicine in the treatment of T2D.