Latrunculin A
Inhibits actin polymerization
概要
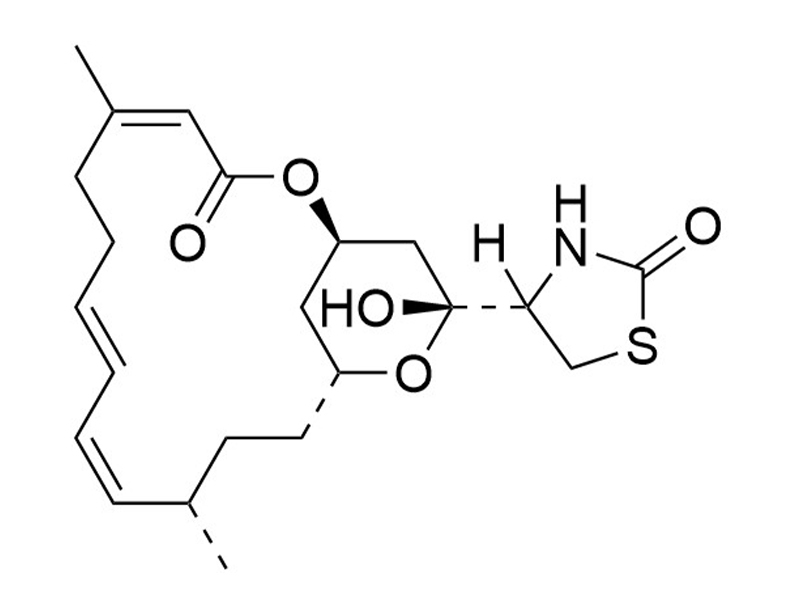
Latrunculin A is a 2-thiazolidinone macrolide isolated from the Red Sea sponge Latrunculia magnifica (Coué et al.). It forms a 1:1 molar complex with monomeric G-actin and inhibits actin polymerization in vitro (Kd = 0.2 μM; Coué et al.). Actins are important for numerous cellular functions such as endocytosis, cell migration, and tumor invasion. This product is supplied as a 100 μg/mL solution in ethanol.
CANCER RESEARCH
· Disrupts actin cytoskeleton in tumor cells (Hayot et al.).
CANCER RESEARCH
· Disrupts actin cytoskeleton in tumor cells (Hayot et al.).
Alternative Names
NSC 613011
Cell Type
Cancer Cells and Cell Lines
Area of Interest
Cancer Research
CAS Number
76343-93-6; 64-17-5
Chemical Formula
C22H31NO5S
Molecular Weight
421.6 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Latrunculin A | 100-0562, 100-0563 | All | English |
| Safety Data Sheet | Latrunculin A | 100-0562, 100-0563 | All | English |
数据及文献
Publications (2)
Toxicology and applied pharmacology 2006 feb
Characterization of the activities of actin-affecting drugs on tumor cell migration.
Abstract
Abstract
Metastases kill 90{\%} of cancer patients. It is thus a major challenge in cancer therapy to inhibit the spreading of tumor cells from primary tumor sites to those particular organs where metastases are likely to occur. Whereas the actin cytoskeleton is a key component involved in cell migration, agents targeting actin dynamics have been relatively poorly investigated. Consequently, valuable in vitro pharmacological tools are needed to selectively identify this type of agent. In response to the absence of any standardized process, the present work aims to develop a multi-assay strategy for screening actin-affecting drugs with anti-migratory potentials. To validate our approach, we used two cancer cell lines (MCF7 and A549) and three actin-affecting drugs (cytochalasin D, latrunculin A, and jasplakinolide). We quantified the effects of these drugs on the kinetics of actin polymerization in tubes (by means of spectrofluorimetry) and on the dynamics of actin cytoskeletons within whole cells (by means of fluorescence microscopy). Using quantitative videomicroscopy, we investigated the actual effects of the drugs on cell motility. Finally, the combined drug effects on cell motility and cell growth were evaluated by means of a scratch-wound assay. While our results showed concordant drug-induced effects on actin polymerization occurring in vitro in test tubes and within whole cells, the whole cell assay appeared more sensitive than the tube assay. The inhibition of actin polymerization induced by cytochalasin D was paralleled by a decrease in cell motility for both cell types. In the case of jasplakinolide, which induces actin polymerization, while it significantly enhanced the locomotion of the A549 cells, it significantly inhibited that of the MCF-7 ones. All these effects were confirmed by means of the scratch-wound assay except of the jasplakinolide-induced effects on MCF-7 cell motility. These later seemed compensated by an additional effect occurring during wound recolonization (possibly acting on the cell growth features). In conclusion, the use of multi-assays with different levels of sophistication and biological relevance is recommended in the screening of new actin-affecting drugs with potentially anti-migratory effects.
FEBS letters 1987 mar
Inhibition of actin polymerization by latrunculin A.
Abstract
Abstract
Latrunculin A, a toxin purified from the red sea sponge Latrunculia magnifica, was found previously to induce striking reversible changes in the morphology of mammalian cells in culture and to disrupt the organization of their microfilaments. We now provide evidence that latrunculin A affects the polymerization of pure actin in vitro in a manner consistent with the formation of a 1:1 molar complex between latrunculin A and G-actin. The equilibrium dissociation constant (Kd) for the reaction in vitro is about 0.2 microM whereas the effects of the drug on cultured cells are detectable at concentrations in the medium of 0.1-1 microM.