LY364947
Activin/BMP/TGF-β pathway inhibitor; Inhibits ALK5
概要
LY364947 is a selective inhibitor of the TGFβ/Activin/NODAL pathway that inhibits ALK5 (IC₅₀ = 59 nM)(Sawyer et al.). LY364947 less effectively inhibits TGFβRII (IC₅₀ = 400 nM), p38 MAPK (IC₅₀ = 740 nM), and mixed lineage kinase-7 (MLK-7; IC₅₀ = 1,400 nM) (Li et al., 2006; Sawyer et al.).
REPROGRAMMING
· In combination with valproic acid, can replace SOX2 in reprogramming of mouse embryonic fibroblasts transduced with OCT4, KLF4 and c-MYC (Ichida et al.).
DIFFERENTIATION
· Blocks chondrogenesis induced by mechanical load in human mesenchymal stem cells (Li et al., 2010).
· Restores the hematopoietic potential of mouse para-aortic splanchnopleural cells deficient for the Evi-1 transcription factor (Sato et al.).
· Impairs definitive endoderm differentiation competence in human embryonic stem (ES) cells (Jaremko et al.).
· Blocks TGF-β-induced endothelial-to-mesenchymal transition of NMuMg mammary epithelial cells or mouse ES cell-derived endothelial cells (Peng et al.; Kokudo et al.).
CANCER RESEARCH
· Suppresses colony-forming ability of mouse and human leukemia-initiating cells cultured with OP-9 stromal cells, and, when combined with imatinib, reduces lethality in a mouse model of chronic myeloid leukemia (Naka et al.).
· Reduces invasiveness of MDA-MB-231 breast cancer cells in a matrigel invasion assay (Shiou et al.).
REPROGRAMMING
· In combination with valproic acid, can replace SOX2 in reprogramming of mouse embryonic fibroblasts transduced with OCT4, KLF4 and c-MYC (Ichida et al.).
DIFFERENTIATION
· Blocks chondrogenesis induced by mechanical load in human mesenchymal stem cells (Li et al., 2010).
· Restores the hematopoietic potential of mouse para-aortic splanchnopleural cells deficient for the Evi-1 transcription factor (Sato et al.).
· Impairs definitive endoderm differentiation competence in human embryonic stem (ES) cells (Jaremko et al.).
· Blocks TGF-β-induced endothelial-to-mesenchymal transition of NMuMg mammary epithelial cells or mouse ES cell-derived endothelial cells (Peng et al.; Kokudo et al.).
CANCER RESEARCH
· Suppresses colony-forming ability of mouse and human leukemia-initiating cells cultured with OP-9 stromal cells, and, when combined with imatinib, reduces lethality in a mouse model of chronic myeloid leukemia (Naka et al.).
· Reduces invasiveness of MDA-MB-231 breast cancer cells in a matrigel invasion assay (Shiou et al.).
Alternative Names
E-616451; HTS 466284; TGF-β RI Kinase Inhibitor
Cell Type
Cancer Cells and Cell Lines, Chondrocytes, Endothelial Cells, Hematopoietic Stem and Progenitor Cells, Mammary Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation, Reprogramming
Area of Interest
Cancer Research, Stem Cell Biology
CAS Number
396129-53-6
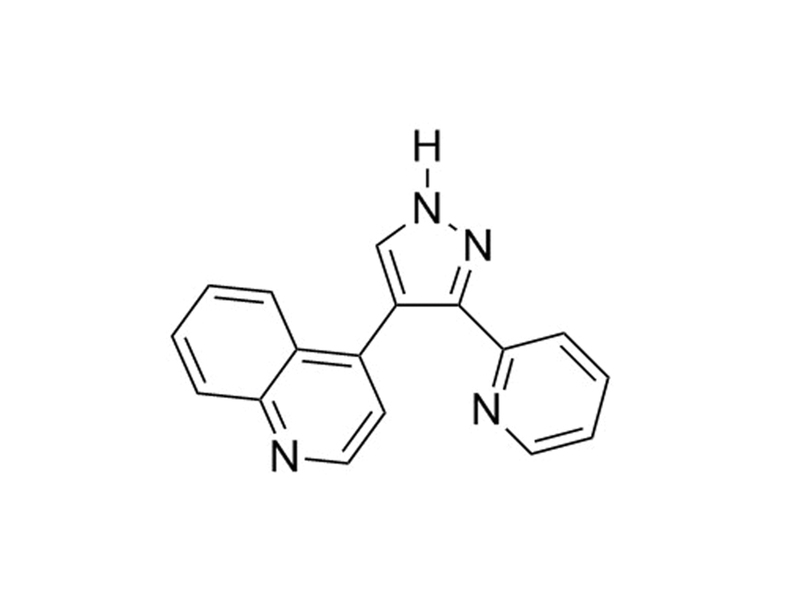
Chemical Formula
C₁₇H₁₂N₄
Molecular Weight
272.3 g/mol
Purity
≥ 98%
Pathway
Activin/Nodal/TGFβ
Target
ALK
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | LY364947 | 72592 | All | English |
| Safety Data Sheet | LY364947 | 72592 | All | English |
数据及文献
Publications (10)
Stem cell research 2013 MAY
Regulation of developmental competence and commitment towards the definitive endoderm lineage in human embryonic stem cells.
Abstract
Abstract
Human embryonic stem cells (hESCs) can self-renew and become all three germ layers. Nodal/Activin signaling specifies developmental status in hESCs: moderate Nodal/Activin signaling maintains pluripotency, while enhancement and inhibition promote definitive endoderm (DE) and neuroectoderm (NE) development, respectively. However, how modulation of Nodal/Activin signaling influences developmental competence and commitment toward specific lineages is still unclear. Here, we showed that enhancement of Nodal/Activin signaling for 4 days was necessary and sufficient to upregulate DE markers, while it diminished the upregulation of NE markers by inhibition of Nodal/Activin signaling. This suggests that after 4 days of enhanced Nodal/Activin signaling, hESCs are committed to the DE lineage and have lost competence toward the NE lineage. In contrast, inhibition of Nodal/Activin signaling using LY364947 for 2 days was sufficient to impair competence toward the DE lineage, although cells were still able to activate LEFTY1 and NODAL, direct targets of Nodal/Activin signaling. Expression analyses indicated that the levels of pluripotency regulators NANOG and POU5F1 were significantly diminished by 2 days of LY364947 treatment, although the expression of NANOG, but not POU5F1, was restored immediately upon Activin A treatment. Thus, downregulation of POU5F1 coincided with the abrogation of DE competence caused by inhibition of Nodal/Activin signaling.
Journal of cellular and molecular medicine 2010 JUN
Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway.
Abstract
Abstract
This study investigated the effect of mechanical load on human mesenchymal stem cell (hMSC) differentiation under different exogenous transforming growth factor-beta1 (TGF-beta(1)) concentrations (0, 1 or 10 ng/ml).The role of the TGF-beta signalling pathway in this process was also studied. Human MSCs were seeded into fibrin-biodegradable polyurethane scaffolds at a cell density of 5 x 10(6) cells per scaffold and stimulated using our bioreactor. One hour of surface motion superimposed on cyclic compression was applied once a day over seven consecutive days. Scaffolds were analysed for gene expression, DNA content and glycosaminoglycan amount. Addition of TGF-beta(1) in the culture medium was sufficient to induce chondrogenesis of hMSCs. Depending on the TGF-beta(1) concentration of the culture medium, mechanical load stimulated chondrogenesis of hMSCs compared to the unloaded scaffolds, with a much stronger effect on gene expression at lower TGF-beta(1) concentrations. With TGF-beta(1) absent in the culture medium, mechanical load stimulated gene transcripts and protein synthesis of TGF-beta(1) and TGF-beta(3). TGF-beta type I receptor inhibitor LY364947 blocked the up-regulation on TGF-beta(1) and TGF-beta(3) production stimulated by mechanical load, and also blocked the chondrogenesis of hMSCs. Taken together, these findings suggest that mechanical load promotes chondrogenesis of hMSCs through TGF-beta pathway by up-regulating TGF-beta gene expression and protein synthesis.
Nature 2010 FEB
TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia.
Abstract
Abstract
Chronic myeloid leukaemia (CML) is caused by a defined genetic abnormality that generates BCR-ABL, a constitutively active tyrosine kinase. It is widely believed that BCR-ABL activates Akt signalling that suppresses the forkhead O transcription factors (FOXO), supporting the proliferation or inhibiting the apoptosis of CML cells. Although the use of the tyrosine kinase inhibitor imatinib is a breakthrough for CML therapy, imatinib does not deplete the leukaemia-initiating cells (LICs) that drive the recurrence of CML. Here, using a syngeneic transplantation system and a CML-like myeloproliferative disease mouse model, we show that Foxo3a has an essential role in the maintenance of CML LICs. We find that cells with nuclear localization of Foxo3a and decreased Akt phosphorylation are enriched in the LIC population. Serial transplantation of LICs generated from Foxo3a(+/+) and Foxo3a(-/-) mice shows that the ability of LICs to cause disease is significantly decreased by Foxo3a deficiency. Furthermore, we find that TGF-beta is a critical regulator of Akt activation in LICs and controls Foxo3a localization. A combination of TGF-beta inhibition, Foxo3a deficiency and imatinib treatment led to efficient depletion of CML in vivo. Furthermore, the treatment of human CML LICs with a TGF-beta inhibitor impaired their colony-forming ability in vitro. Our results demonstrate a critical role for the TGF-beta-FOXO pathway in the maintenance of LICs, and strengthen our understanding of the mechanisms that specifically maintain CML LICs in vivo.
Cell stem cell 2009 NOV
A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog.
Abstract
Abstract
The combined activity of three transcription factors can reprogram adult cells into induced pluripotent stem cells (iPSCs). However, the transgenic methods used for delivering reprogramming factors have raised concerns regarding the future utility of the resulting stem cells. These uncertainties could be overcome if each transgenic factor were replaced with a small molecule that either directly activated its expression from the somatic genome or in some way compensated for its activity. To this end, we have used high-content chemical screening to identify small molecules that can replace Sox2 in reprogramming. We show that one of these molecules functions in reprogramming by inhibiting Tgf-beta signaling in a stable and trapped intermediate cell type that forms during the process. We find that this inhibition promotes the completion of reprogramming through induction of the transcription factor Nanog.
Journal of cell science 2008 OCT
Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells.
Abstract
Abstract
Epithelial-mesenchymal transition (EMT) plays important roles in various physiological and pathological processes, and is regulated by signaling pathways mediated by cytokines, including transforming growth factor beta (TGFbeta). Embryonic endothelial cells also undergo differentiation into mesenchymal cells during heart valve formation and aortic maturation. However, the molecular mechanisms that regulate such endothelial-mesenchymal transition (EndMT) remain to be elucidated. Here we show that TGFbeta plays important roles during mural differentiation of mouse embryonic stem cell-derived endothelial cells (MESECs). TGFbeta2 induced the differentiation of MESECs into mural cells, with a decrease in the expression of the endothelial marker claudin 5, and an increase in expression of the mural markers smooth muscle alpha-actin, SM22alpha and calponin, whereas a TGFbeta type I receptor kinase inhibitor inhibited EndMT. Among the transcription factors involved in EMT, Snail was induced by TGFbeta2 in MESECs. Tetracycline-regulated expression of Snail induced the differentiation of MESECs into mural cells, whereas knockdown of Snail expression abrogated TGFbeta2-induced mural differentiation of MESECs. These results indicate that Snail mediates the actions of endogenous TGFbeta signals that induce EndMT.
Cancer science 2008 JUL
Evi-1 promotes para-aortic splanchnopleural hematopoiesis through up-regulation of GATA-2 and repression of TGF-b signaling.
Abstract
Abstract
Evi-1 is a zinc-finger transcriptional factor whose inappropriate expression leads to leukemic transformation in mice and humans. Recently, it has been shown that Evi-1 regulates proliferation of hematopoietic stem/progenitor cells at embryonic stage via GATA-2 up-regulation; however, detailed mechanisms underlying Evi-1-mediated early hematopoiesis are not fully understood. We therefore evaluated hematopoietic potential of Evi-1 mutants using a cultivation system of murine para-aortic splanchnopleural (P-Sp) regions, and found that both the first zinc finger domain and the acidic domain were required for Evi-1-mediated hematopoiesis. The hematopoietic potential of Evi-1 mutants was likely to be related to its ability to up-regulate GATA-2 expression. We also showed that the decreased colony forming capacity of Evi-1-deficient P-Sp cells was successfully recovered by inhibition of TGF-b signaling, using ALK5 inhibitor or retroviral transfer of dominant-negative-type Smad3. Our findings suggest that Evi-1 promotes hematopoietic stem/progenitor expansion at the embryonic stage through up-regulation of GATA-2 and repression of TGF-beta signaling.