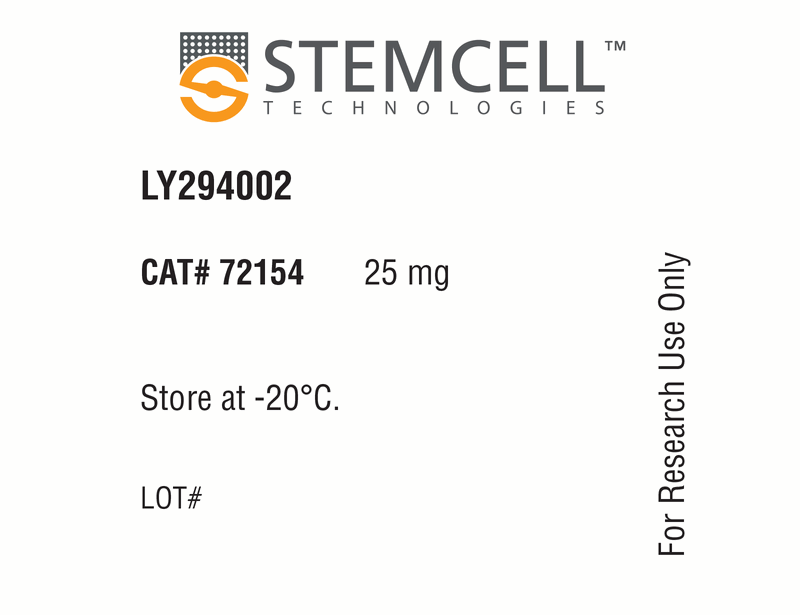
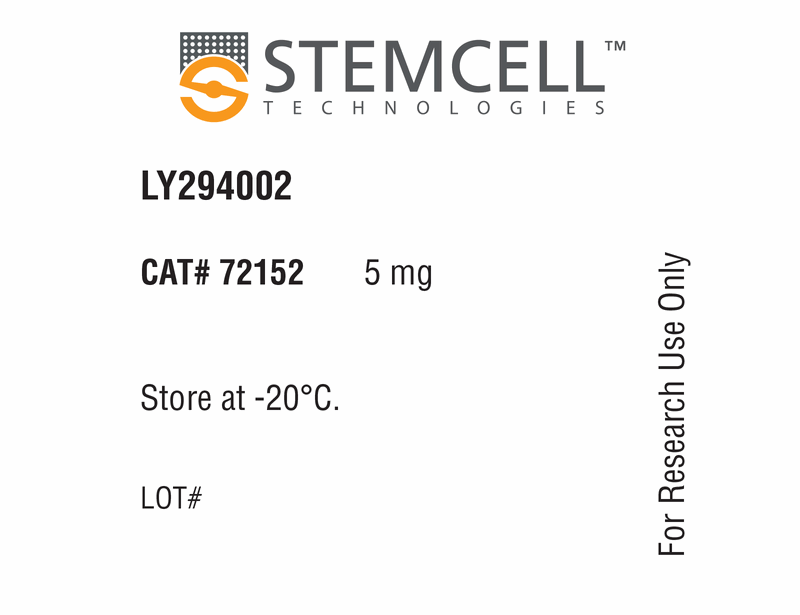
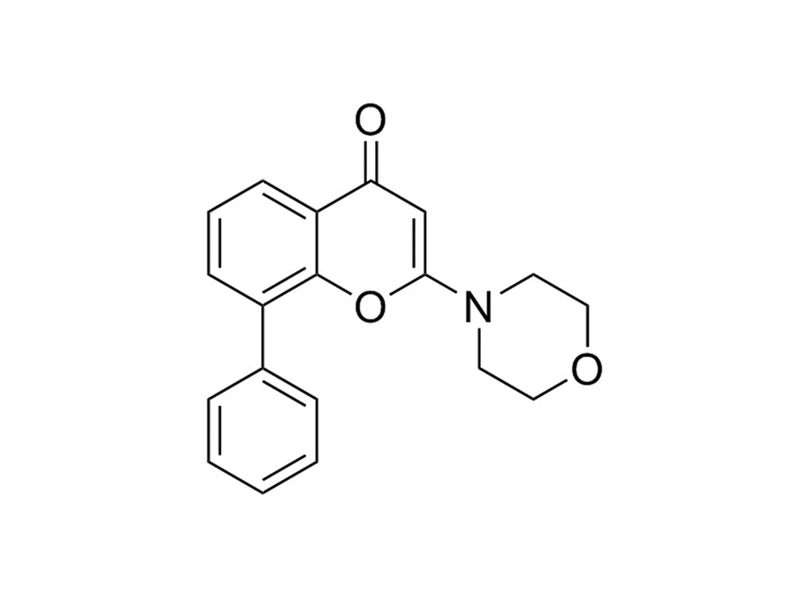
LY294002
PI3K/AKT pathway inhibitor; Inhibits PI3K
概要
LY294002 is a PI3K inhibitor that has greater potency and selectivity than quercetin, the structure on which it is based. LY294002 inhibits PI3K (IC₅₀ = 1.4 µM) and also shows activity against CK2, but not PI4K, EGFR, PDGFR, MAPK, PKA, or PKC. (Davies et al., Vlahos et al.)
MAINTENANCE AND SELF-RENEWAL
· Suppresses proliferation and self-renewal of mouse embryonic stem (ES) cells (Lianguzova et al., Paling et al.).
DIFFERENTIATION
· Promotes differentiation to insulin-producing cells from mouse ES cells (Hori et al.).
· Inhibits myotube formation from myoblasts (Coolican et al., Jiang et al.).
MAINTENANCE AND SELF-RENEWAL
· Suppresses proliferation and self-renewal of mouse embryonic stem (ES) cells (Lianguzova et al., Paling et al.).
DIFFERENTIATION
· Promotes differentiation to insulin-producing cells from mouse ES cells (Hori et al.).
· Inhibits myotube formation from myoblasts (Coolican et al., Jiang et al.).
Alternative Names
Not applicable
Cell Type
Endoderm, PSC-Derived, Myogenic Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human, Mouse, Rat, Non-Human Primate, Other
Application
Differentiation
Area of Interest
Neuroscience, Stem Cell Biology
CAS Number
154447-36-6
Chemical Formula
C₁₉H₁₇NO₃
Molecular Weight
307.3 g/mol
Purity
≥ 98%
Pathway
PI3K/AKT
Target
PI3K
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | LY294002 | 72152, 72154 | All | English |
| Safety Data Sheet | LY294002 | 72152, 72154 | All | English |
数据及文献
Publications (7)
Cell 2015 JUL
Deterministic Restriction on Pluripotent State Dissolution by Cell-Cycle Pathways.
Abstract
Abstract
During differentiation, human embryonic stem cells (hESCs) shut down the regulatory network conferring pluripotency in a process we designated pluripotent state dissolution (PSD). In a high-throughput RNAi screen using an inclusive set of differentiation conditions, we identify centrally important and context-dependent processes regulating PSD in hESCs, including histone acetylation, chromatin remodeling, RNA splicing, and signaling pathways. Strikingly, we detected a strong and specific enrichment of cell-cycle genes involved in DNA replication and G2 phase progression. Genetic and chemical perturbation studies demonstrate that the S and G2 phases attenuate PSD because they possess an intrinsic propensity toward the pluripotent state that is independent of G1 phase. Our data therefore functionally establish that pluripotency control is hardwired to the cell-cycle machinery, where S and G2 phase-specific pathways deterministically restrict PSD, whereas the absence of such pathways in G1 phase potentially permits the initiation of differentiation.
Cell biology international 2007 APR
Phosphoinositide 3-kinase inhibitor LY294002 but not serum withdrawal suppresses proliferation of murine embryonic stem cells.
Abstract
Abstract
Mouse embryonic stem (mES) cells have short duration of their cell cycle and are capable of proliferating in the absence of growth factors. To find out which signaling pathways contribute to the regulation of the mES cell cycle, we used pharmacological inhibitors of MAP and PI3 kinase cascades. The MAP kinase inhibitors as well as serum withdrawal did not affect mES cell cycle distribution, whereas the inhibitor of PI3K activity, LY294002, induced accumulation of cells in G(1) phase followed by apoptotic cell death. Serum withdrawal also causes apoptosis, but it does not change the content and activity of cell cycle regulators. In contrast, in mES cells treated with LY294002, the activities of Cdk2 and E2F were significantly decreased. Interestingly, LY294002had a much stronger effect on cell cycle distribution in low serum conditions, implying that serum can promote G(1)--textgreaterS transition of mES cells by a LY294002-resistant mechanism. Thus, proliferation of mES cells is maintained by at least two separate mechanisms: a LY294002-sensitive pathway, which is active even in the absence of serum, and LY294002-resistant, but serum-dependent, pathway.
The Journal of biological chemistry 2004 NOV
Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling.
Abstract
Abstract
The maintenance of murine embryonic stem (ES) cell self-renewal is regulated by leukemia inhibitory factor (LIF)-dependent activation of signal transducer and activator of transcription 3 (STAT3) and LIF-independent mechanisms including Nanog, BMP2/4, and Wnt signaling. Here we demonstrate a previously undescribed role for phosphoinositide 3-kinases (PI3Ks) in regulation of murine ES cell self-renewal. Treatment with the reversible PI3K inhibitor, LY294002, or more specific inhibition of class I(A) PI3K via regulated expression of dominant negative Deltap85, led to a reduction in the ability of LIF to maintain self-renewal, with cells concomitantly adopting a differentiated morphology. Inhibition of PI3Ks reduced basal and LIF-stimulated phosphorylation of PKB/Akt, GSK3alpha/beta, and S6 proteins. Importantly, LY294002 and Deltap85 expression had no effect on LIF-induced phosphorylation of STAT3 at Tyr(705), but did augment LIF-induced phosphorylation of ERKs in both short and long term incubations. Subsequently, we demonstrate that inhibition of MAP-Erk kinases (MEKs) reverses the effects of PI3K inhibition on self-renewal in a time- and dose-dependent manner, suggesting that the elevated ERK activity observed upon PI3K inhibition contributes to the functional response we observe. Surprisingly, upon long term inhibition of PI3Ks we observed a reduction in phosphorylation of beta-catenin, the target of GSK-3 action in the canonical Wnt pathway, although no consistent alterations in cytosolic levels of beta-catenin were observed, indicating this pathway is not playing a major role downstream of PI3Ks. Our studies support a role for PI3Ks in regulation of self-renewal and increase our understanding of the molecular signaling components involved in regulation of stem cell fate.
The Biochemical journal 2000 OCT
Specificity and mechanism of action of some commonly used protein kinase inhibitors.
Abstract
Abstract
The specificities of 28 commercially available compounds reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases have been examined against a large panel of protein kinases. The compounds KT 5720, Rottlerin and quercetin were found to inhibit many protein kinases, sometimes much more potently than their presumed targets, and conclusions drawn from their use in cell-based experiments are likely to be erroneous. Ro 318220 and related bisindoylmaleimides, as well as H89, HA1077 and Y 27632, were more selective inhibitors, but still inhibited two or more protein kinases with similar potency. LY 294002 was found to inhibit casein kinase-2 with similar potency to phosphoinositide (phosphatidylinositol) 3-kinase. The compounds with the most impressive selectivity profiles were KN62, PD 98059, U0126, PD 184352, rapamycin, wortmannin, SB 203580 and SB 202190. U0126 and PD 184352, like PD 98059, were found to block the mitogen-activated protein kinase (MAPK) cascade in cell-based assays by preventing the activation of MAPK kinase (MKK1), and not by inhibiting MKK1 activity directly. Apart from rapamycin and PD 184352, even the most selective inhibitors affected at least one additional protein kinase. Our results demonstrate that the specificities of protein kinase inhibitors cannot be assessed simply by studying their effect on kinases that are closely related in primary structure. We propose guidelines for the use of protein kinase inhibitors in cell-based assays.
Proceedings of the National Academy of Sciences of the United States of America 1998 NOV
An essential role of phosphatidylinositol 3-kinase in myogenic differentiation.
Abstract
Abstract
The oncogene p3k, coding for a constitutively active form of phosphatidylinositol 3-kinase (PI 3-kinase; EC 2.7.1.137), strongly enhances myogenic differentiation in cultures of chicken-embryo myoblasts. It increases the size of the myotubes and induces elevated levels of the muscle-specific proteins MyoD, myosin heavy chain, creatine kinase, and desmin. Inhibition of PI 3-kinase activity with LY294002 or with dominant-negative mutants of PI 3-kinase interferes with myogenic differentiation and with the induction of muscle-specific genes. PI 3-kinase is therefore an upstream mediator for the expression of the muscle-specific genes and is both necessary and rate-limiting for the process of myogenesis.
The Journal of biological chemistry 1997 MAR
The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways.
Abstract
Abstract
It is well established that mitogens inhibit differentiation of skeletal muscle cells, but the insulin-like growth factors (IGFs), acting through a single receptor, stimulate both proliferation and differentiation of myoblasts. Although the IGF-I mitogenic signaling pathway has been extensively studied in other cell types, little is known about the signaling pathway leading to differentiation in skeletal muscle. By using specific inhibitors of the IGF signal transduction pathway, we have begun to define the signaling intermediates mediating the two responses to IGFs. We found that PD098059, an inhibitor of mitogen-activated protein (MAP) kinase kinase activation, inhibited IGF-stimulated proliferation of L6A1 myoblasts and the events associated with it, such as phosphorylation of the MAP kinases and elevation of c-fos mRNA and cyclin D protein. Surprisingly, PD098059 caused a dramatic enhancement of differentiation, evident both at a morphological (fusion of myoblasts into myotubes) and biochemical level (elevation of myogenin and p21 cyclin-dependent kinase inhibitor expression, as well as creatine kinase activity). In sharp contrast, LY294002, an inhibitor of phosphatidylinositol 3-kinase, and rapamycin, an inhibitor of the activation of p70 S6 kinase (p70(S6k)), completely abolished IGF stimulation of L6A1 differentiation. We found that p70(S6k) activity increased substantially during differentiation, and this increase was further enhanced by PD098059. Our results demonstrate that the MAP kinase pathway plays a primary role in the mitogenic response and is inhibitory to the myogenic response in L6A1 myoblasts, while activation of the phosphatidylinositol 3-kinase/p70(S6k) pathway is essential for IGF-stimulated differentiation. Thus, it appears that signaling from the IGF-I receptor utilizes two distinct pathways leading either to proliferation or differentiation.