Human Recombinant Activin A
Activin A
概要
Activin A is a member of the transforming growth factor beta (TGF-β) family of proteins produced by many cell types throughout development (Gurdon et al.). It is a disulfide-linked homodimer (two beta-A chains) that binds to heteromeric complexes of a type I (Act RI-A and Act RI-B) and a type II (Act RII-A and Act RII-B) serine-threonine kinase receptor (Attisano et al.). Activins primarily signal through SMAD2/3 proteins to regulate a variety of functions, including cell proliferation, differentiation, wound healing, apoptosis, and metabolism (McDowell et al.). Activin A maintains the undifferentiated state of human embryonic stem cells (James et al.; Xiao et al.) and also facilitates differentiation of human embryonic stem cells into definitive endoderm (D’Amour et al.).
Subtype
Cytokines
Alternative Names
Activin beta-A chain, EDF, Erythroid differentiation protein, FRP, FSH-releasing protein, INHBA, Inhibin beta-A chain, Inhibin beta-1
Cell Type
Endoderm, PSC-Derived, Mesoderm, PSC-Derived, Other, Pluripotent Stem Cells
Species
Human
Area of Interest
Epithelial Cell Biology, Stem Cell Biology
Molecular Weight
26.2 kDa
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Human Recombinant Activin A | 78001, 78001.1, 78001.2, 78001.3 | All | English |
| Safety Data Sheet | Human Recombinant Activin A | 78001, 78001.1, 78001.2, 78001.3 | All | English |
数据及文献
Data
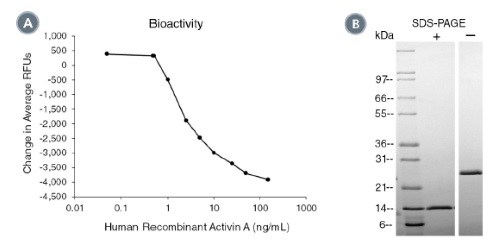
(A) The biological activity of Human Recombinant Activin A was tested by its ability to induce cytotoxicity of MPC-11 cells. Cytotoxicity
was measured after 48 hours of culture using a fluorometric assay method. The EC50 is defined as the effective concentration of the
growth factor at which cell death is at 50% of maximum. The EC50 in the above example is 1.9 - 2.9 ng/mL.
(B) 1 μg of Human Recombinant Activin A was resolved with SDS-PAGE under reducing (+) and non-reducing (-) conditions visualized by
Coomassie Blue staining. Human Recombinant Activin A is a homodimer with a predicted molecular mass of 26.2 kDa.
Publications (2)
International Journal of Molecular Sciences 2016 JAN
Effect of chromatin structure on the extent and distribution of DNA double strand breaks produced by ionizing radiation; comparative study of hESC and differentiated cells lines
Abstract
Abstract
Chromatin structure affects the extent of DNA damage and repair. Thus, it has been shown that heterochromatin is more protective against DNA double strand breaks (DSB) formation by ionizing radiation (IR); and that DNA DSB repair may proceed differently in hetero- and euchromatin regions. Human embryonic stem cells (hESC) have a more open chromatin structure than differentiated cells. Here, we study the effect of chromatin structure in hESC on initial DSB formation and subsequent DSB repair. DSB were scored by comet assay; and DSB repair was assessed by repair foci formation via 53BP1 antibody staining. We found that in hESC, heterochromatin is confined to distinct regions, while in differentiated cells it is distributed more evenly within the nuclei. The same dose of ionizing radiation produced considerably more DSB in hESC than in differentiated derivatives, normal human fibroblasts; and one cancer cell line. At the same time, the number of DNA repair foci were not statistically different among these cells. We showed that in hESC, DNA repair foci localized almost exclusively outside the heterochromatin regions. We also noticed that exposure to ionizing radiation resulted in an increase in heterochromatin marker H3K9me3 in cancer HT1080 cells, and to a lesser extent in IMR90 normal fibroblasts, but not in hESCs. These results demonstrate the importance of chromatin conformation for DNA protection and DNA damage repair; and indicate the difference of these processes in hESC.
Stem Cells International 2012 JAN
Stimulation of cultured h9 human embryonic stem cells with thyroid stimulating hormone does not lead to formation of thyroid-like cells.
Abstract
Abstract
The sodium-iodine symporter (NIS) is expressed on the cell membrane of many thyroid cancer cells, and is responsible for the radioactive iodine accumulation. However, treatment of anaplastic thyroid cancer is ineffective due to the low expression of NIS on cell membranes of these tumor cells. Human embryonic stem cells (ESCs) provide a potential vehicle to study the mechanisms of NIS expression regulation during differentiation. Human ESCs were maintained on feeder-independent culture conditions. RT-qPCR and immunocytochemistry were used to study differentiation marker expression, (125)I uptake to study NIS function. We designed a two-step protocol for human ESC differentiation into thyroid-like cells, as was previously done for mouse embryonic stem cells. First, we obtained definitive endoderm from human ESCs. Second, we directed differentiation of definitive endoderm cells into thyroid-like cells using various factors, with thyroid stimulating hormone (TSH) as the main differentiating factor. Expression of pluripotency, endoderm and thyroid markers and (125)I uptake were monitored throughout the differentiation steps. These approaches did not result in efficient induction of thyroid-like cells. We conclude that differentiation of human ESCs into thyroid cells cannot be induced by TSH media supplementation alone and most likely involves complicated developmental patterns that are yet to be understood.